The reaction of aldehydes and ketones with secondary amines produces enamines. Enamines are amines with a double bond on the adjacent carbon (alkene + amine = enamine):

Let’s go over the mechanism of this transformation to see how it works.
In the previous post, we saw that aldehydes and ketones react with primary amines, in mildly acidic conditions, forming an imine (a Schiff base). The reaction starts with a nucleophilic addition of the amine on the carbonyl group, and after a series of proton transfers and elimination of H2O, an imine is formed:

Enamine Formation Mechanism
If you have already learned this mechanism, then there is not much changing here. It is only the last step that makes the difference in forming an enamine instead of an imine. And the reason for this is the lack of a proton that is removed from the iminium ion in the last step of imine formation
We will put these mechanisms next to each other, going down to compare each step one by one:
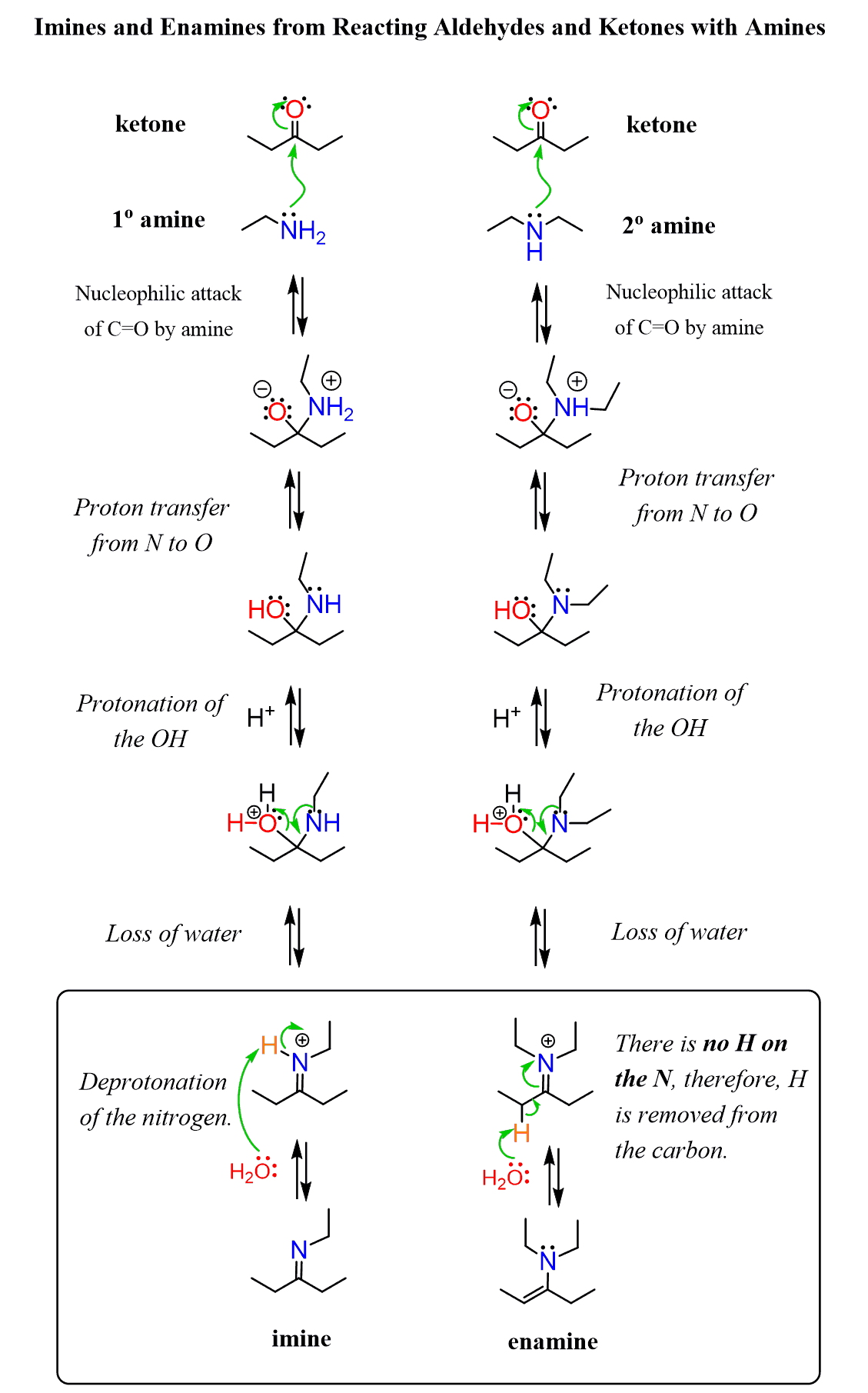
So, in the last step, the proton is removed not from the nitrogen since there is not one but rather from the adjacent carbon in an E2 fashion, forming an enamine.
Like imine or acetal formation, enamine formation is reversible and is driven to completion by the removal of water or other products. Enamines are also formed as a mixture of (E) and (Z) isomers when applicable.
Some More Thoughts on Imines and Enamines
There is a good question you may be wondering – why wouldn’t enamines be formed in the reaction of primary amines with aldehydes or ketones?
The answer is just like ketones are more stable than enols, imines are more stable than enamines, and whenever possible (that is, when there is a hydrogen on the enamine nitrogen), it is largely converted to an imine:

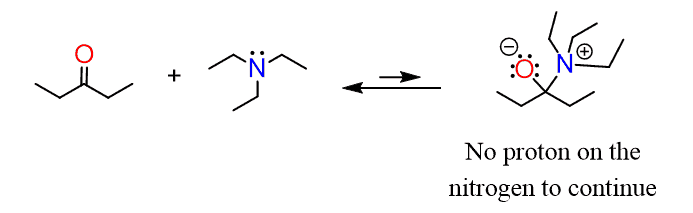
Another question might be the reaction of tertiary amines with aldehydes and ketones. And, the problem here is that there is no proton on the nitrogen to even form the aminoalcohol (carbinolamine), and the reaction is reversed back once the initial nucleophilic attack occurs:
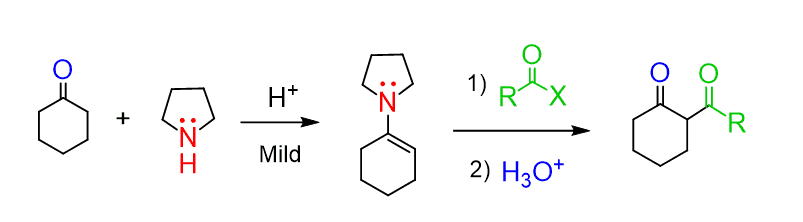
Just like imines, enamines also have a lot of applications in organic synthesis. They can be used for preparing amines by reductive amination with sodium cyanoborohydride (NaCNBH3) or in the Stork enamine synthesis as a mild and efficient way of alkylating the ɑ-carbon:
Check Also
- Nomenclature of Aldehydes and Ketones
- How to Name a Compound with Multiple Functional Groups
- Preparation of Aldehydes and Ketones
- Nucleophilic Addition to Carbonyl Groups
- Reduction of Aldehydes and Ketones
- Reactions of Aldehydes and Ketones with Water
- Reactions of Aldehydes and Ketones with Alcohols: Acetals and Hemiacetals
- Acetals as Protecting Groups for Aldehydes and Ketones
- Formation and Reactions of Imines and Enamines
- Reductive Amination
- Acetal Hydrolysis Mechanism
- Imine and Enamine Hydrolysis Mechanism
- Hydrolysis of Acetals, Imines, and Enamines-Practice Problems
- Reaction of Aldehydes and Ketones with CN, Cyanohydrin Formation
- The Wittig Reaction: Examples and Mechanism
- The Wittig Reaction: Practice Problems
- Aldehydes and Ketones to Carboxylic Acids
- Reactions of Aldehydes and Ketones – Practice Problems
- Aldehydes and Ketones Reactions Practice Quiz
- Reactions Map of Aldehydes
- Reactions Map of Ketones

In the text you said ”The answer is just like ketones are more stable than enols, imines are more stable than enamines and whenever possible (that is when there is a hydrogen on the imine nitrogen) the enamine is largely converted to an imine:”
Shouldn’t it be ”(that is when there is a hydrogen on the ENAMINE nitrogen) the … ”?
Yes, this is correct – the enamine of a primary amine converts to an imine because of the hydrogen.
Thank you!
Why secondary amines give enamines and not N,N-acetal analogous to OR and SR nulceophiles, which give O,O- and S,S-acetals by condensation to carbonyl compounds?
Hi there,
I think the question you are asking can be rephrased as “why another molecule of the amine is not attacking the intermediate iminium ion as a nucleophile thus adding to the C=N double bond?”.
Let me mention first that N,N-acetals do exist and it’s not something impossible to occur. However, there are couple of things to consider. One is the sterics which pertains to both the amine and the alkyl groups of the iminium ion. Specifically for secondary amines, you should know that they are basic rather nucleophilic because of their bulkiness. So, instead of attacking the C=N, it will deprotonate the a position and form an enamine. Consider also that even if it acted as a nucleophile and attacked the C=N bond, a geminal diamine with two alkyl groups would’ve formed which is sterically quite hindered and will easily break back. All you’d need is protonate one of the amines and second one expels it like it happens in the hydrolysis of acetals.
Now, if the iminium ion is derived from formaldehyde, then N,N-acetals are more likely to form because: 1) the iminium ion is less hindered and 2) there are no electron-donating alkyl groups that suppress the electrophilicity of the C=N carbon.
For further reading, Bruckner’s Advanced Organic Chemistry talks about this in more detail.