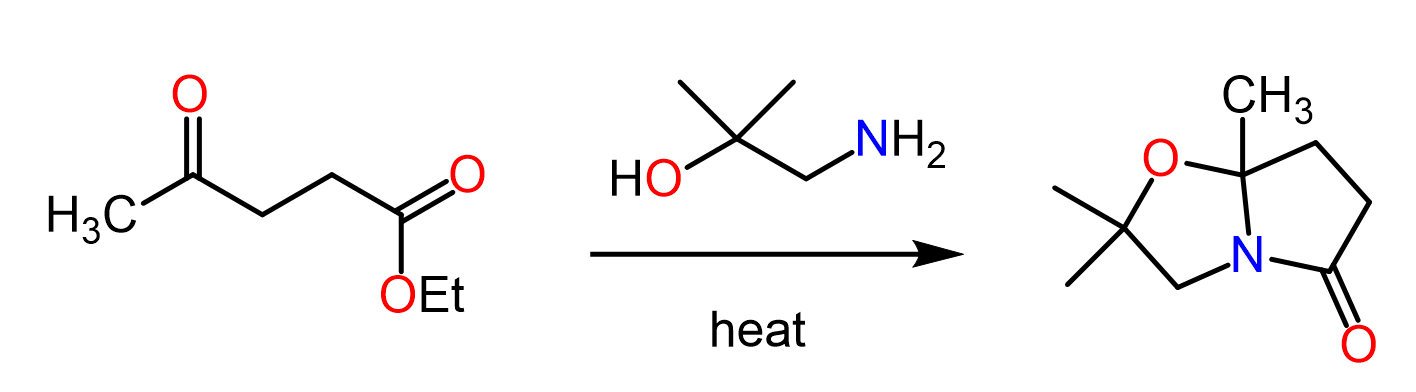
Esters can be converted into primary, secondary and tertiary amides by an aminolysis reaction with ammonia, primary amine and a secondary amine respectively:

The reaction goes by a nucleophilic addition-elimination mechanism and alkoxy groups (RO–), being poor leaving groups, make this method not as practical as, for example, the reaction of acyl chlorides with amines. And in general, acyl chlorides require milder conditions for nucleophilic acyl substitution than the alternative approaches do. Remember the limitations of Fischer esterification such as the need for high temperatures and nucleophilicity of the alcohol.
Going back to the reaction between esters and amines; let’s understand how it happens and why this conversion is possible anyway.
In the first, we have a nucleophilic addition of the amine to the carbonyl and with a plenty of amine in the reaction mixture, the nitrogen is quickly deprotonated forming a negatively charged tetrahedral intermediate:
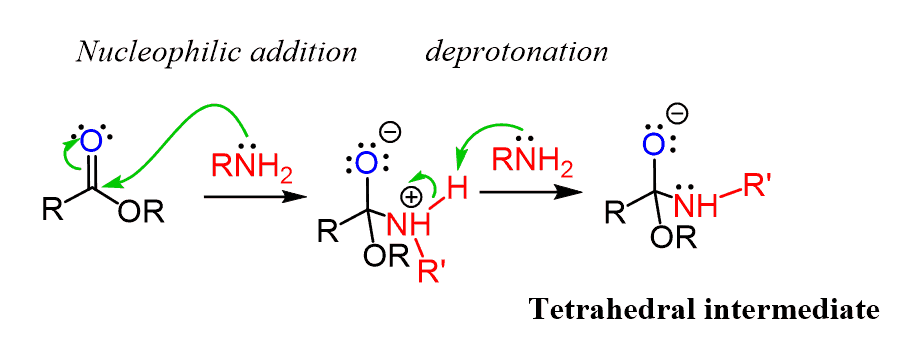
In order to restore the C=O double, either the alkoxy (RO–) or the (RNH–) must be expelled.
Which one do you think is a better leaving group?
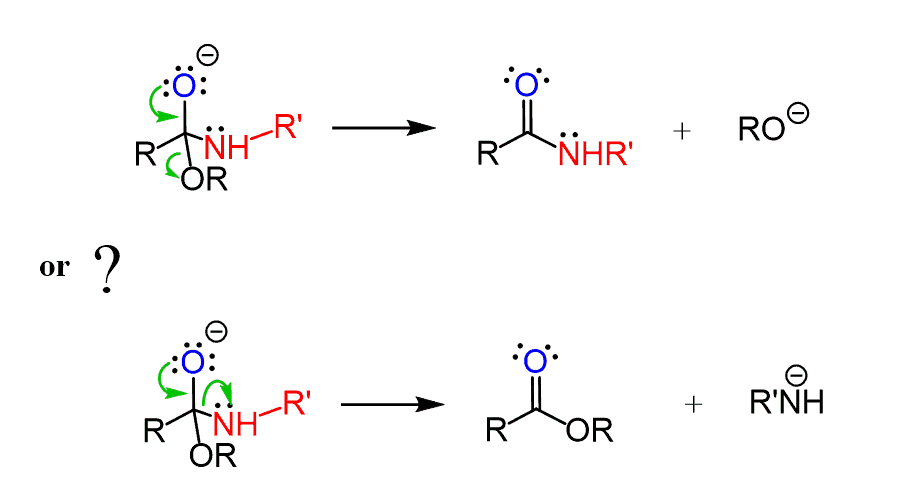
Comparing the pKa values of alcohols (16) and amines (38), we know that alkoxy groups are much weaker bases and therefore better leaving groups than a conjugate base of an amine. So, when the lone pairs on the oxygen moving down to restore the C=O bond, the alkoxy group is kicked out producing an amide:
Let’s put these together to get a complete mechanism of ester reaction with amines:

Now, even though the alkoxy is a better leaving group than a conjugate base of an amine, it still is a very poor leaving. Because of this, the aminolysis of esters is not an efficient way of preparing amides. Again, acyl chlorides would be the better option in most cases.