Acid-Catalyzed Addition of Alcohols and Alkoxymercuration
Alkenes can be converted to ethers by reacting them with alcohols in the presence of acid or using Hg(OAc)2 catalyst.

You may wonder why use a toxic mercury catalyst if it gives the same result as the addition of alcohols to alkenes under acidic conditions. It is a valid question considering that both gave the same product, and even the regiochemical outcome is the same – the alcohol ended up on the more substituted carbon of the double bond. In other words, we can say that they both give “Markovnikov” addition.
The answer to this equation is that using the mercury catalyst prevents any possible rearrangements in electrophilic additions to alkenes. For example, when 3-methylbut-1-ene is reacted with methanol under acidic conditions, 2-methoxy-2-methylbutane is obtained instead of 2-methoxy-3-methylbutane, which would be the Markovnikov product:
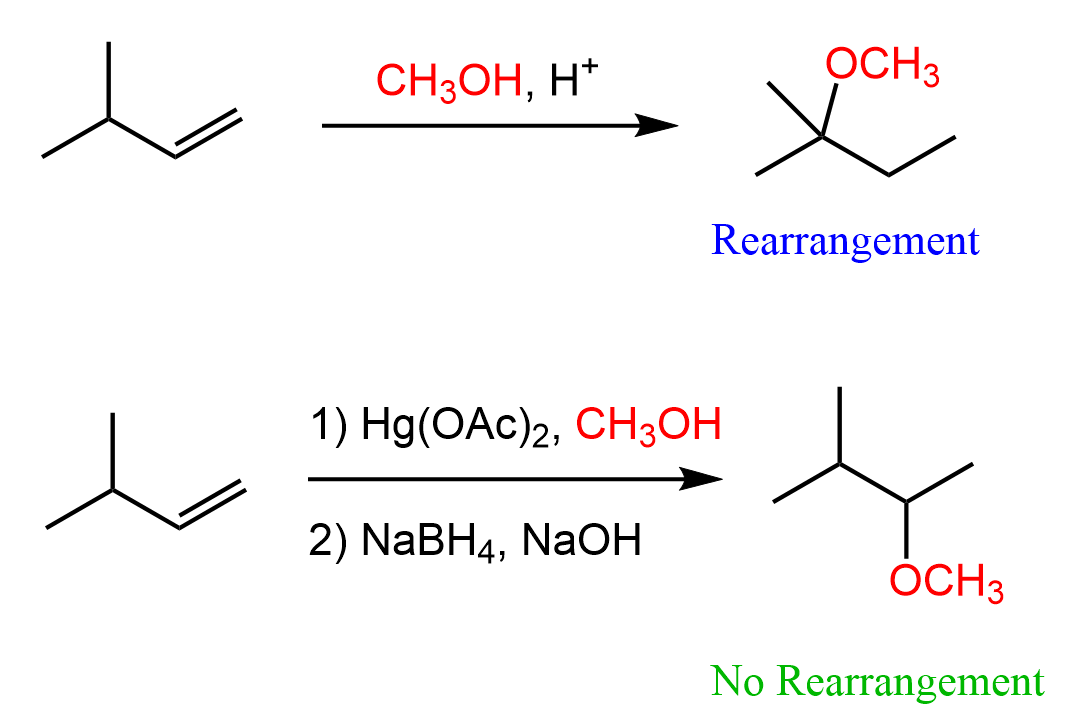
This is due to the rearrangement of the secondary carbocation intermediate to the more stable tertiary carbocation:

This drawback can be overcome by using the Hg(OAc)2 catalyst instead of the acid. Unlike the protonation, Hg(OAc)2 does not convert the alkene into a carbocation, but rather a reactive intermediate called mercurinium ion is formed. Due to the ring strain and positive charge, the mercurinium ion is very susceptible to a nucleophilic attack, but importantly, it does not undergo a rearrangement. This means the Markovnikov addition of the OH group to the double bond can be achieved without worrying about possible rearrangements:

This reaction is called alkoxymercuration, and it is a variation of the oxymercuration-demercuration reaction, which is used as an alternative to the acid-catalyzed addition of alcohols to alkenes to avoid possible rearrangements. You can find more about these reactions, their mechanism, regio- and stereochemistry in the linked articles, as this post is focused on the approaches for preparing ethers.
Comparing Williamson Synthesis to Alkoxymercuration
Another question to address here is how these methods compare to the Williamson ether synthesis, which employs the SN2 reaction between alkyl halides and alkoxy ions (the conjugate base of an alcohol):

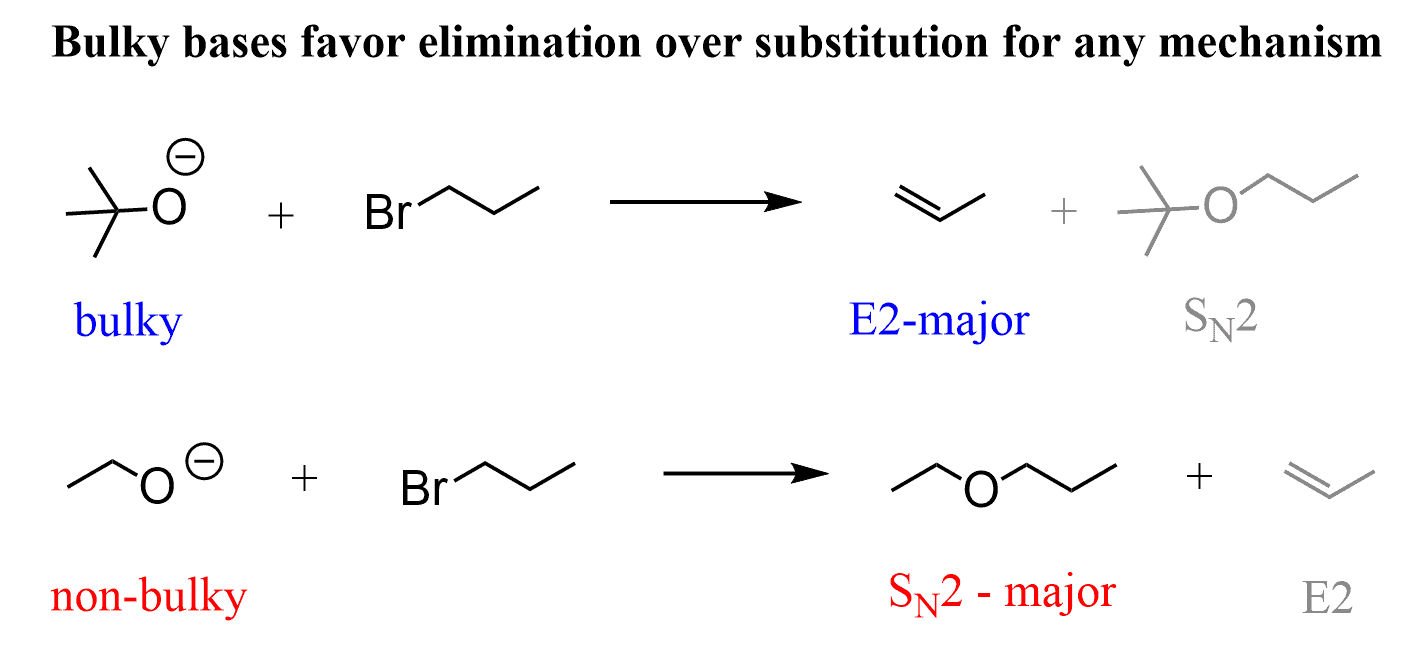
Williamson ether synthesis is a great method for preparing ethers. However, as an SN2 reaction, it has hit certain limits when bulky reactants are used (check the article deciding between SN1, SN2, E1, and E2 reactions).
To summarize what we discussed, alkenes can be converted to ethers by their reaction with alcohols in the presence of acid or mercury catalysts.
- The alkoxymercuration is used to prevent possible rearrangements in the acid-catalyzed addition of alcohols.
Ethers can also be prepared by the Williamson reaction between alkyl halides and alkoxy ions.
- The drawback of the Williamson ether synthesis is its limitation to relatively non-bulky reactants-primary and secondary at best.
Check Also
- Markovnikov’s Rule with Practice Problems
- Acid-Catalyzed Hydration of Alkenes with Practice Problems
- Oxymercuration-Demercuration
- Addition of Alcohols to Alkenes
- Free-Radical Addition of HBr: Anti-Markovnikov Addition
- Hydroboration-Oxidation: The Mechanism
- Hydroboration-Oxidation of Alkenes: Regiochemistry and Stereochemistry with Practice Problems
- Halogenation of Alkenes and Halohydrin Formation
- The Stereochemistry of Alkene Addition Reactions
- Cis product in an anti Addition Reaction of Alkenes
- Ozonolysis of Alkenes with Practice Problems
- Syn Dihydroxylation of Alkenes with KMnO4 and OsO4
- Anti Dihydroxylation of Alkenes with MCPBA and Other Peroxides with Practice Problems
- Oxidative Cleavage of Alkenes with KMno4 and O3
- Alkene Reactions Practice Problems
- Changing the Position of a Double Bond
- Changing the Position of a Leaving Group
- Alkenes Multi-Step Synthesis Practice Problems
- Alkene Addition Reactions Practice Quiz
