Acetals are organic compounds in which a carbon atom is bonded to two alkoxy groups (-OR).
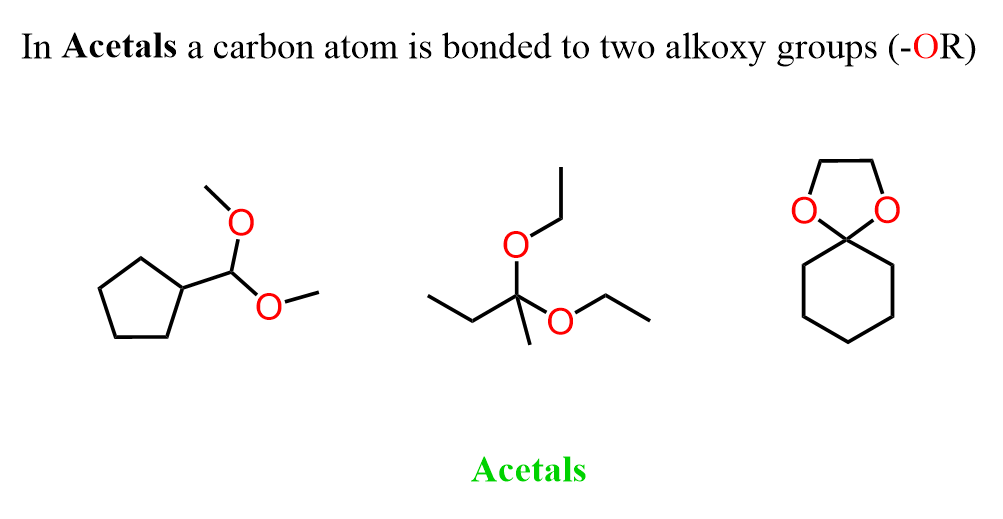
They don’t look much like a carbonyl compound, so why do we need to talk about them in the chapter on aldehydes and ketones? The short answer to this is that acetals are prepared from aldehydes and ketones by reacting them with alcohols in acidic conditions.
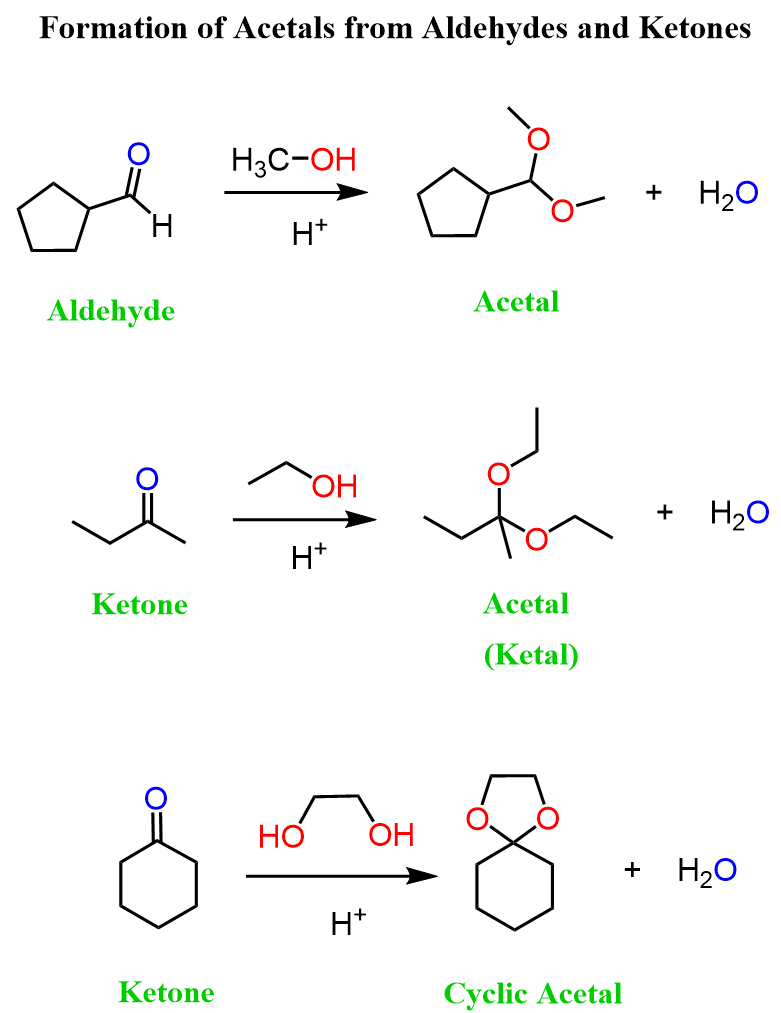
The formation of acetals is an example of a nucleophilic addition reaction to the carbonyl group. In the big picture, think of this as replacing the carbonyl oxygen (C=O) with two alkoxy groups (OR). In other words, we’re swapping one double bond for two single bonds:
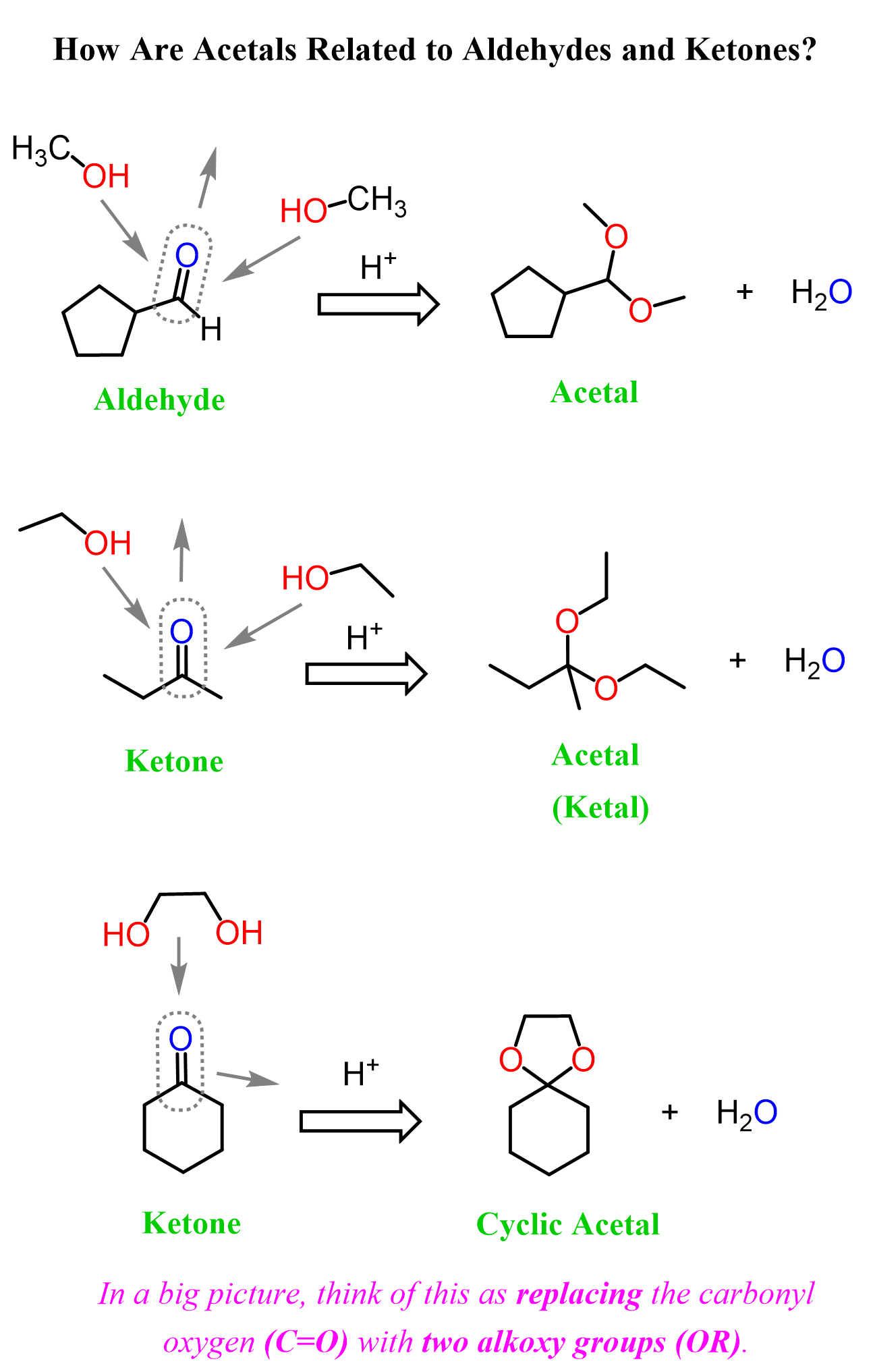
So, how does this happen? What is the mechanism of acetal formation from aldehydes and ketones? Yeah, let’s make this more serious and draw some curved arrows instead of swapping the carbonyl with alkoxy groups.
The Mechanism of Acetal Formation
The reaction is carried out in slightly acidic conditions, so let’s first understand why we need the acid here. We know from SN1 and SN2 reactions that alcohols are weak nucleophiles, so to make them attack another molecule, we need to make the latter greatly electrophilic. The other molecule here is the carbonyl group of the aldehyde or ketone. The C=O bond is polarized because of the higher electronegativity of the oxygen, thus making the carbon partially positively charged and prone to nucleophilic attacks:
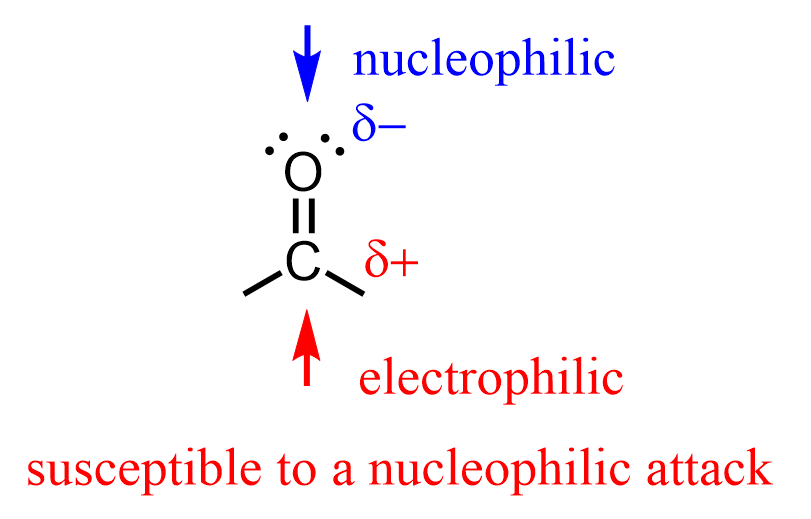
Now, it is not as though the alcohol cannot attack the carbonyl group without having an acid catalyst – it can and it does. However, we add the acid to protonate it, therefore making the carbonyl more polarized and the C=O carbon more electron-deficient.
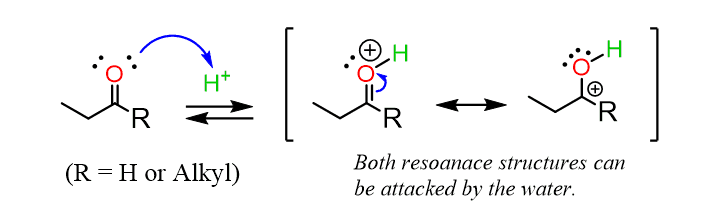
This is a common strategy in many nucleophilic addition reactions to carbonyl compounds, so take note, as you are going to see these types of reactions very often in the upcoming couple of chapters.
Going back to the mechanism of acetal formation, we know that the first step is a protonation, after which the nucleophilic attack of the alcohol to the carbonyl occurs. The resulting compound is called a hemiacetal, which, as the name suggests, is a half-acetal, and it only has one alkoxy group compared with the two in acetals:

Hemiacetals are quite unstable, and they have no significant applications in organic synthesis, so we won’t focus much on them today.
In the next step, the OH group of the hemiacetal is protonated and converted into a good leaving group:
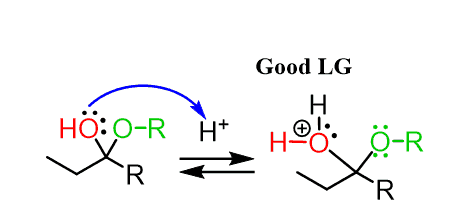
This may look ready to be attacked by another alcohol molecule, kicking out H2O.
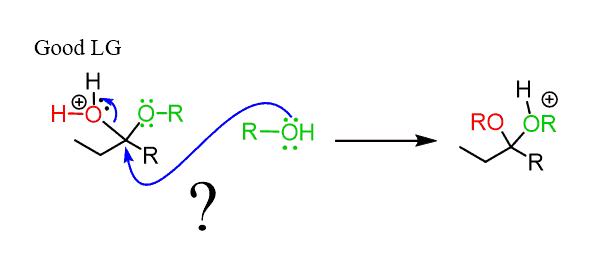
However, notice that the carbon is sterically hindered and therefore, no SN2 can occur here.
Instead, the reaction goes by an SN1 mechanism where the leaving group is first expelled by the lone pairs of the neighboring oxygen, which forms another oxonium intermediate readily available to react with an alcohol nucleophile:
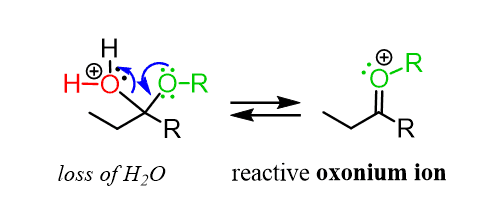
After the nucleophilic attack, we have the deprotonation of the oxonium intermediate, generating an acetal:
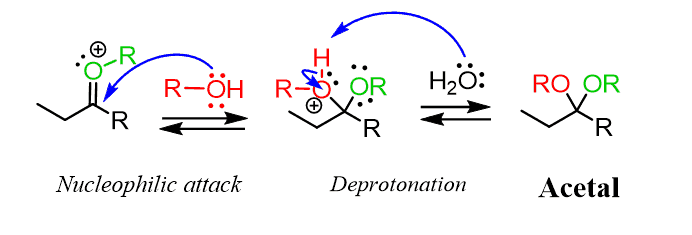
Let’s put this mechanism of acid-catalyzed formation of acetals in a nice summary:

How are Acetals Isolated if the Reaction is at Equilibrium?
Just like the hydration of aldehydes and ketones, the equilibrium generally favors reactants rather than products, except for some simple or more reactive aldehydes. This has to do with the electronic and steric effects, which we covered in the acid-catalyzed hydration of aldehydes and ketones.
There is, however, an important difference to consider when comparing the formation of hydrates and acetal!
Hydrates are prepared in aqueous solutions, which means the water is in an enormous excess.
The synthesis of acetals, on the other hand, produces water as a by-product. This is a great advantage since the equilibrium can be shifted to the right by removing the water as it is formed and forcing the completion of the reaction:
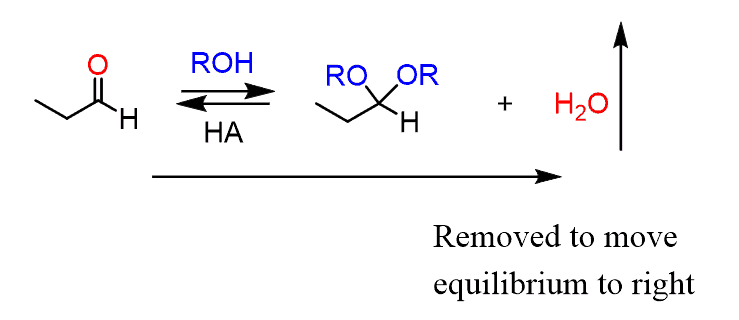
For this, either a drying agent or a special distillation setup (Dean-Stark trap) can be used.
Acetals and Carbohydrates
An important class of acetals is the glycosides. These are the acetals of monosaccharides, which are the simplest carbohydrate molecules. In their open-chain form, sugars like glucose contain an aldehyde functional group, which makes them reactive toward alcohols under acidic conditions.
For example, the treatment of glucose with methanol and HCl leads to the formation of two isomeric glycosides.
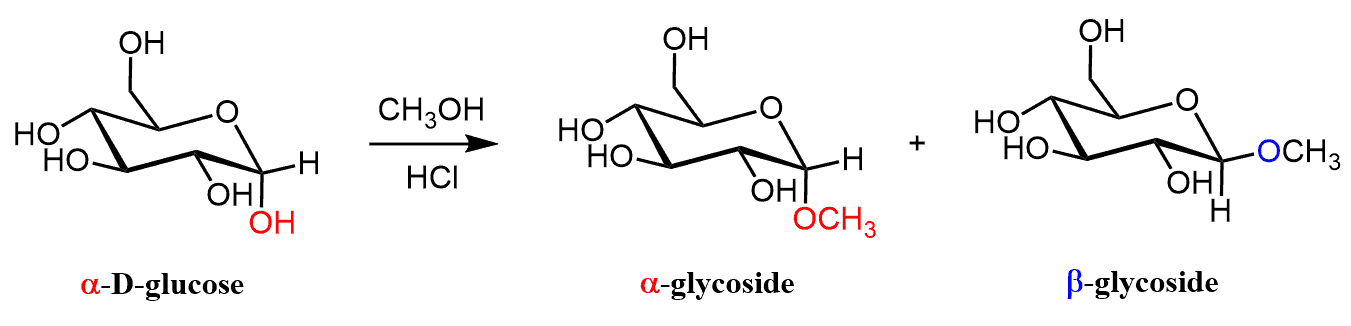
This happens because glucose exists in equilibrium between its open-chain aldehyde form and its cyclic hemiacetal form. The acid catalyzes the reaction between the anomeric carbon (the carbon originally part of the carbonyl group) and methanol, converting the hemiacetal into a full acetal, or glycoside.
What’s interesting is that this reaction creates two different stereoisomers, known as the α- and β-anomers, depending on the position of the methoxy group at the anomeric carbon. These glycosides are much more stable than hemiacetals because acetals do not easily revert to the open-chain form under neutral or basic conditions.
Glycosides are also biologically significant and appear in many natural products such as antibiotics, cardiac drugs, and plant metabolites.
Cyclic Acetals
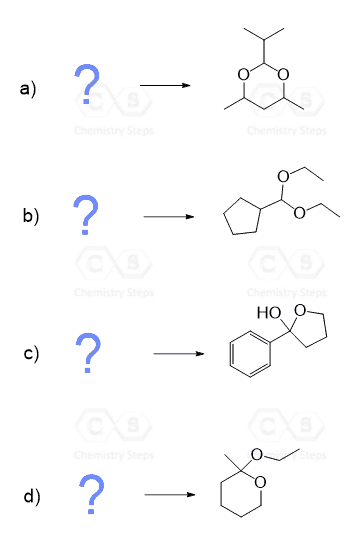
Notice again that acetals contain two alkoxy groups, and therefore, two equivalents of alcohol are reacted with the aldehyde or ketone to produce them.
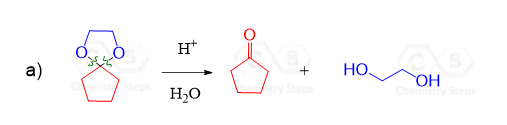
These two equivalents of the OH group can also be incorporated by a diol – an alcohol that contains two OH groups. This reaction produces a cyclic acetal, which is especially favored when five- or six-membered rings are formed:

The mechanism of this reaction is the same as what we saw for the formation of regular acetals. Can you draw it? Simply use the OH of the diol instead of the second equivalent of the alcohol.
Cyclic acetals are very useful as protecting groups for aldehydes and ketones in basic conditions. For example, we can selectively reduce an ester group in the presence of an aldehyde or a ketone using the acetal protecting group:

Remember, aldehydes and ketones are more reactive than esters, and they are reduced easier than esters, so LiAlH4 would reduce both groups to alcohols if the acetal protecting group was not used. More details will be covered in the next post.
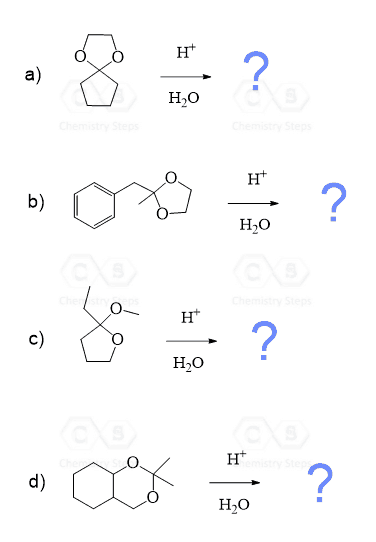
Notice also that the acetal group is formed and hydrolyzed using acid catalysis. Like in the case of water, amines, and other nucleophiles, the addition reactions of aldehydes are often reversible, and the equilibrium is pushed forward or back by adjusting the conditions, such as temperature and the concentration of reactants.
We have a separate post on the hydrolysis of acetals, so feel free to check that out too. You can try drawing a mechanism for the hydrolysis of acetals and compare it to what we have there.
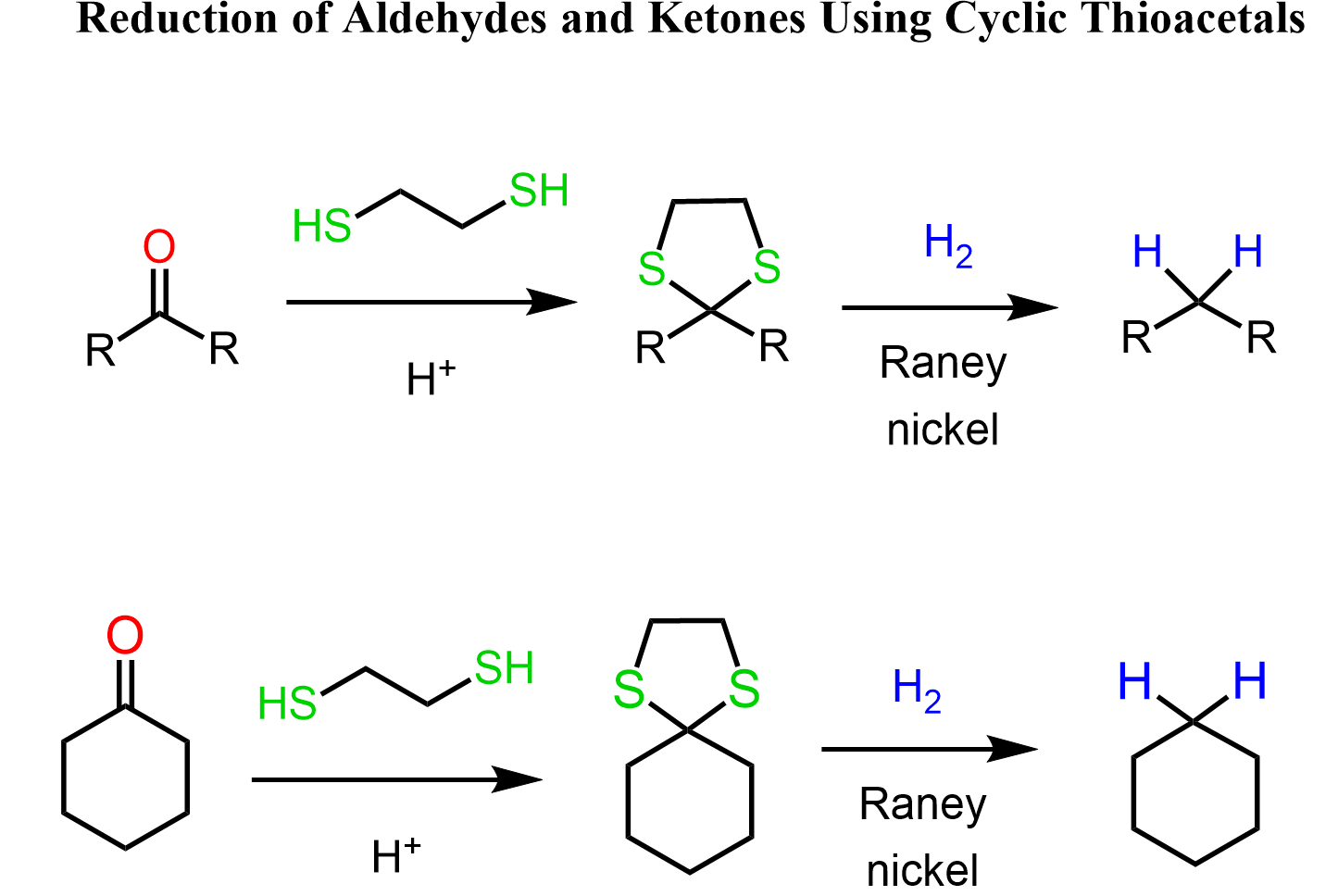
Cyclic Thioacetals
Cyclic thioacetals might look like an odd variation of the acetals we’ve been talking about, but they come with some differences and advantages compared to the oxygen analogs.
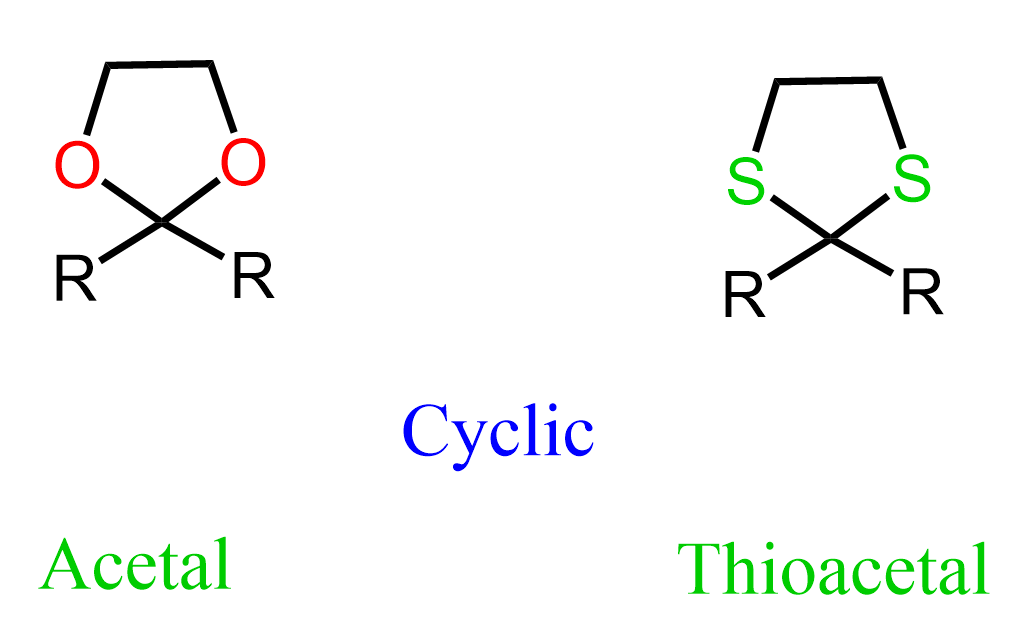
Just like regular acetals, they’re formed by reacting aldehydes or ketones with dithiols under acidic conditions.
However, cyclic thioacetals are a bit unique, and things get interesting: once we’ve masked the carbonyl as a cyclic thioacetal, you can take advantage of its reactivity. For example, we can treat it with Raney nickel to reduce the carbonyl to a CH2 group, effectively removing the oxygen and forming a simple hydrocarbon.
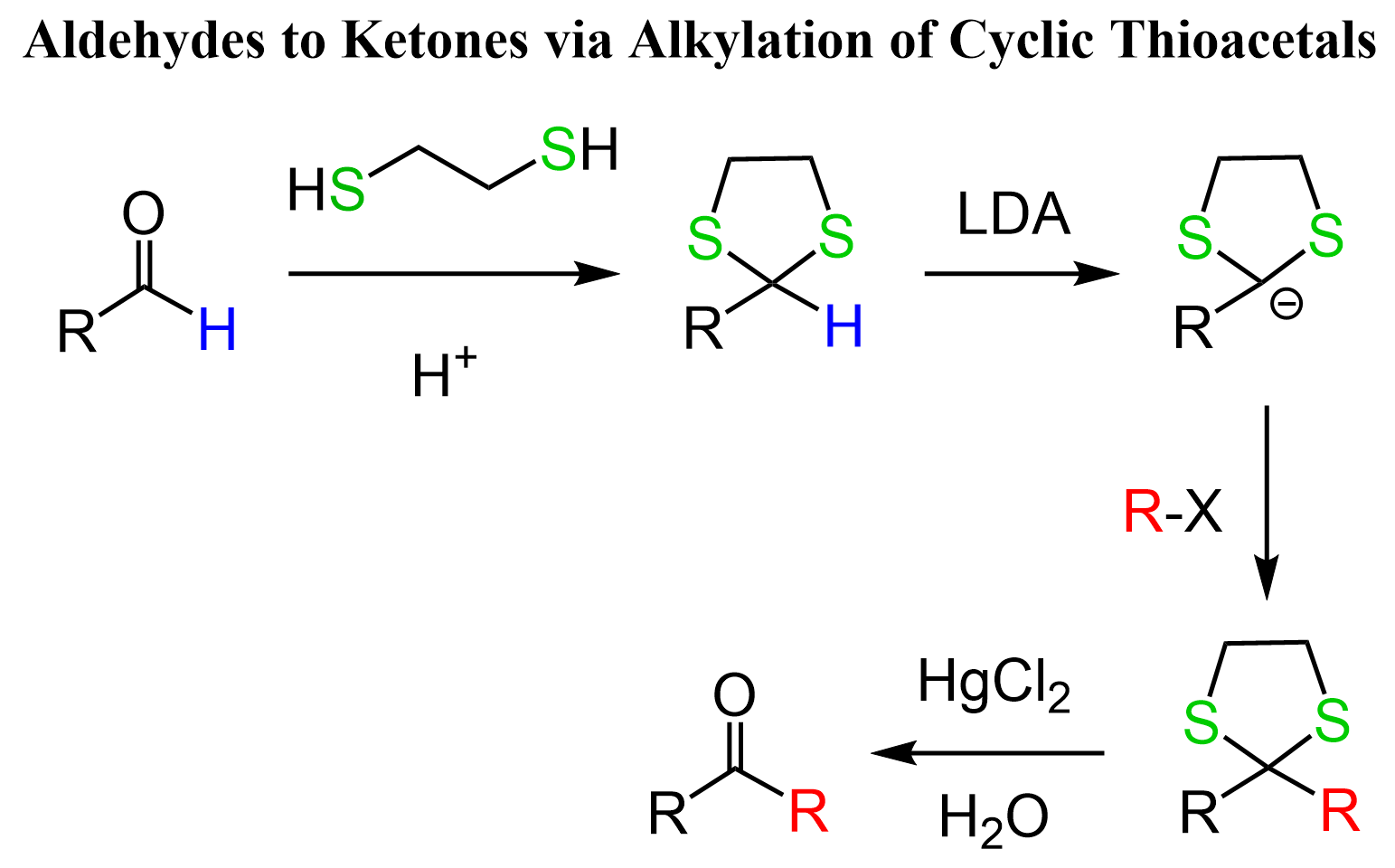
Another interesting feature of cyclic thioacetals is the increased acidity of the carbonyl C-H proton. This makes them a useful way of alkylating aldehydes since they decrease the pKa of the carbonyl protons from ~45 to ~32, and we can deprotonate them with strong bases such as LDA or organolithiums, forming a powerful carbon nucleophile that can react, for example, with alkyl halides. This is a great way of converting an aldehyde to a ketone:
Summarizing Acetals
To summarize what we discussed about acetals, remember first that they are formed by reacting aldehydes and ketones with alcohols and mild acidic conditions. The transformation is overall two additions of the alcohol to the carbonyl, and intermediate hemiacetals are formed after the first addition.
Remember that acetals are also removed under acidic conditions, and it is simply about adjusting concentrations.
A specific type of acetals is cyclic acetals, which are formed when diols are used, and these are used as the main protecting group of aldehydes and ketones in basic conditions, such as when using Grignard, organolithium, or reducing agents.
The sulfur analog, cyclic thioacetals, can be used for reducing the carbonyl to C–H or alkylating the carbonyl carbon, thus converting aldehydes to ketones.