Nucleophilic Additions to the Carbonyl Group
In this chapter on aldehydes and ketones, we begin to see a new type of mechanism originating from the polar nature of the C=O carbonyl bond. When we say “different,” that is mainly compared to the substitution and elimination reactions covered in Organic Chemistry I. Because of this polarity, various nucleophiles tend to attack the carbon atom, as it bears a partial positive charge. These are all nucleophilic addition reactions, which we can visualize in this short mechanistic illustration:

Check out the linked article to read more about the general pattern of nucleophilic addition reactions to carbonyl groups, as this is what we are going to see a lot in this and the upcoming chapters on carboxylic acid derivatives and enolate chemistry.
Formation of Imines and Enamines
Today, we will focus on the addition of primary and secondary amines to aldehydes and ketones.
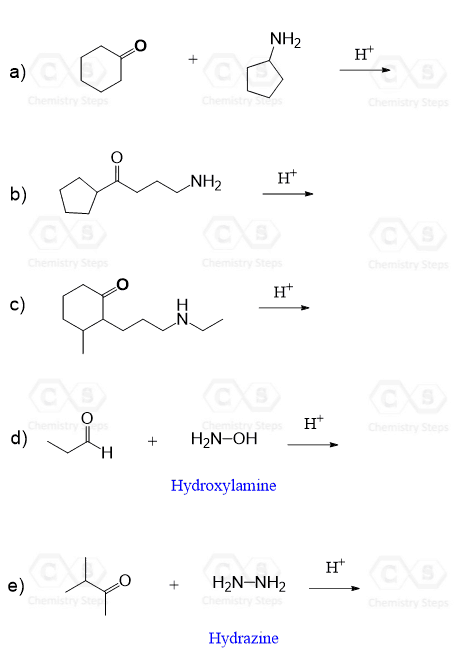
The products of the addition reactions of primary amines are imines, also known as Schiff bases, compounds containing a carbon-nitrogen double bond (C=N), where the nitrogen is connected to a hydrogen or an organic group (alkyl or aryl chain).
The reaction of secondary amines produces enamines, and what is common is that they are both formed via an iminium intermediate, which has two sites for undergoing a deprotonation:

Notice that the lone pair is provided to the positively charged nitrogen atom by deprotonation, and whether the deprotonation occurs on the nitrogen or a neighboring carbon atom from the aldehyde or the ketone fragment, an imine or an enamine is formed. We will talk about this in more detail when discussing the mechanisms for imine and enamine formation, but for now, let’s put a few examples together for the formation of imines and enamines:

What About Ammonia and Tertiary Amines?
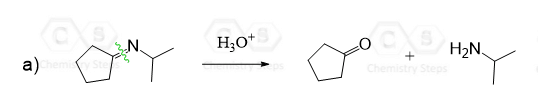
Tertiary amines do not participate in these types of addition reactions, as the alkyl groups make them too bulky, and they are mainly used as organic bases. In addition, even if the nucleophilic attack occurs, a positively charged nitrogen atom is formed, which has no hydrogen atom to lose and get stabilized. So, remember, tertiary amines do not form stable derivatives when reacted with aldehydes or ketones.
Recall from SN2 and E2 reactions that bulkiness does not affect basicity, as, unlike the electrophilic carbon in substitution reactions, the β-hydrogens can still be accessed by the bulky base.
Notice also that imines formed from ammonia are unstable, but can be detected in solution (from Claydon, Organic Chemistry).
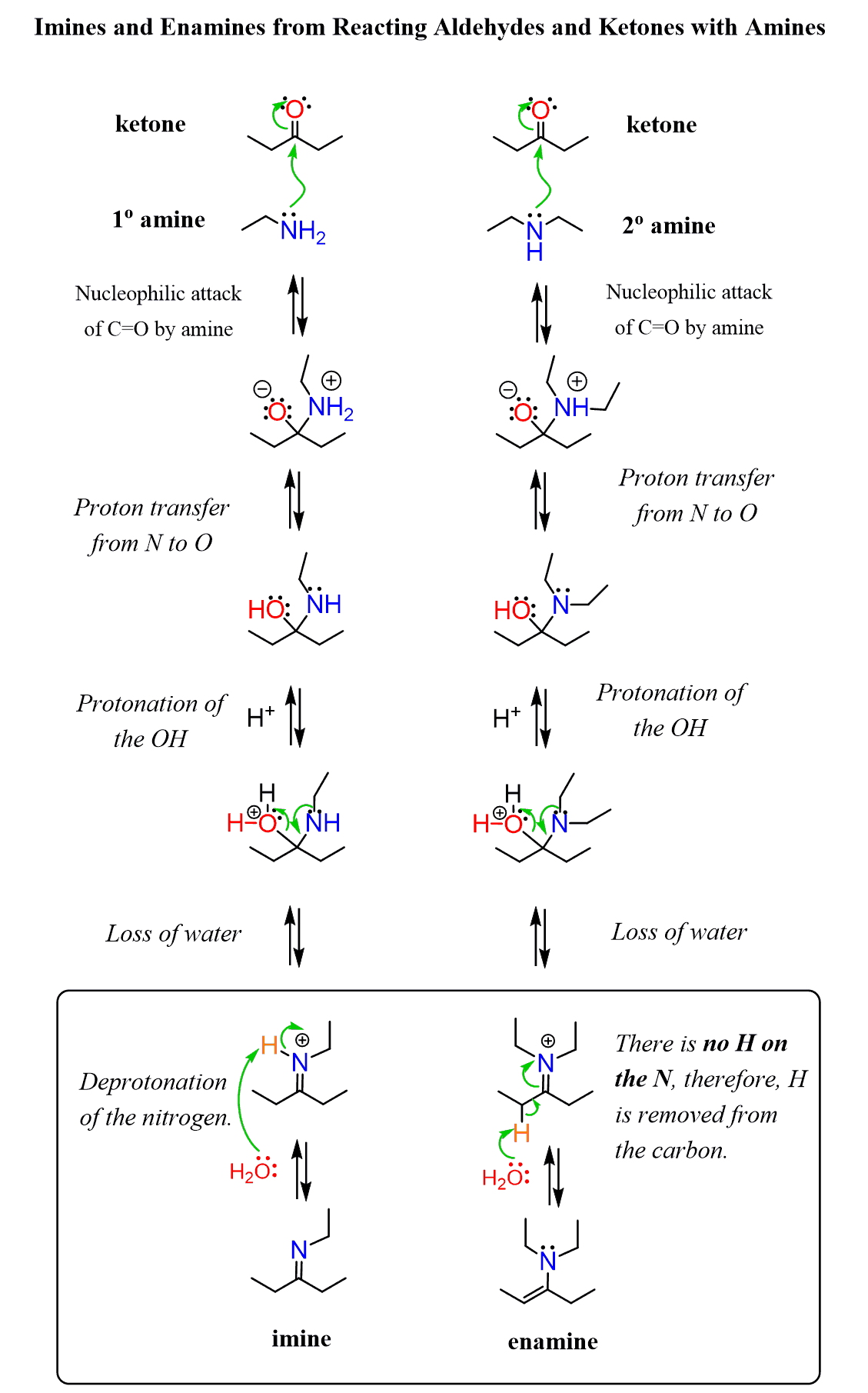
Formation of Imines: The Mechanism
As mentioned earlier, this is a typical nucleophilic addition to the carbonyl group, so the first step is the nucleophilic attack of the nitrogen, forming a C–N bond. After this, a couple of proton transfers occur, converting the OH into a good leaving group, thus facilitating the formation of the C=N double bond.

For a better description, we say this is an addition-elimination reaction, where the nucleophile adds to the carbonyl, and the term “elimination” refers to the loss of water (OH group) or other units such as in the case of acid chlorides (next chapter).

We also have a separate post on the general features of addition-elimination reactions, so check that out to get a better feel for these types of mechanisms – there are many of them in the upcoming chapters as well.
Conditions for Imine Formation
The reaction is carried out in slightly acidic conditions, and the role of the acid is to protonate the carbonyl, thus activating it more toward the nucleophilic addition of the amine.
So, in the first step, we can also show the protonation of the carbonyl group, followed by the nucleophilic addition of the amine.

You may wonder: How come the amine is not protonated first, thus losing its nucleophilicity?
And the answer is it is, but not all of it gets protonated. The small amount of unprotonated amine is enough to initiate the reaction and keep it moving forward, for example, by using the protonated amine as a source of proton transfer in the following steps.
In general, keep in mind that this is a reversible reaction, and the equilibrium is shifted forward by removing the water as it forms. Therefore, when imines are treated with water (in large excess), the reverse reaction occurs, and the corresponding carbonyl compound is formed. More details on this a little later.
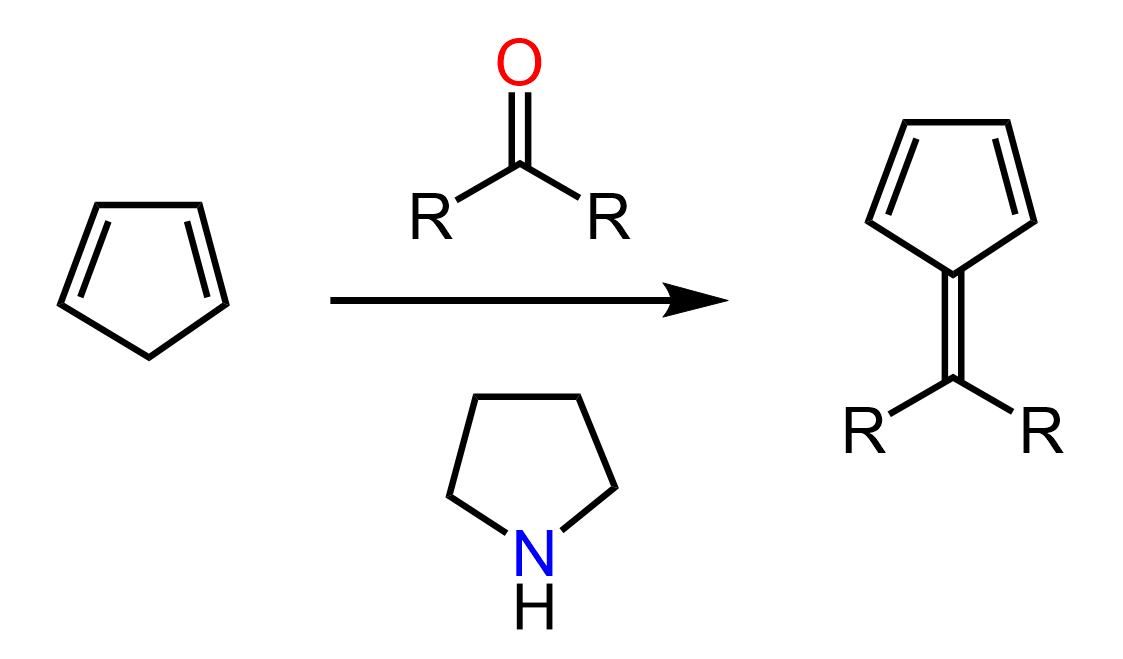
Why Do Secondary Amines Form Enamines?
We mentioned at the beginning of this discussion that secondary amines are still good nucleophiles, and they do react with carbonyl compounds, including aldehydes and ketones. The only difference and the reason enamines, rather than imines, are formed in this reaction is the lack of a second hydrogen on the secondary nitrogen atom. Having both an amino group and a C=C double bond, the resulting compound is called an enamine:

Notice that when the OH group is expelled, a positively charged compound called an iminium ion is formed.
As you may guess from the structure, iminium ions are very electrophilic, so they get stabilized by deprotonation of either the nitrogen (primary amines) or a β-carbon (secondary amines). In the case of primary amines, the nitrogen is deprotonated forming an imine, whereas the lack of this nitrogen proton switches the deprotonation to the neighboring carbon, and a new C=C π bond is formed.
The Mechanism of Enamine Formation
Let’s place the mechanisms of imine and enamine formation side by side to highlight the point of deprotonation where they differ:
Imines and Enamines are Not Unrelated
We said that the intermediate iminium ion formed from primary amines is deprotonated at the nitrogen, while, in the case of secondary amines, it is the neighboring carbon that loses a proton. Fair enough, but this does not answer another question, which is: why wouldn’t enamines be formed in the reaction of primary amines with aldehydes or ketones? Why can’t the elimination on a beta carbon still occur?
Once again, the answer is that it does occur (let’s say at least it can), but you need to know that imines and enamines are not unrelated. In fact, they are the analog of keto-enol tautomerism (KET) of carbonyl compounds.

Importantly, just like ketones are more stable than enols, imines are more stable than enamines, and whenever possible (that is when there is a hydrogen on the enamine nitrogen), it is largely converted to an imine. So, both pathways of deprotonating the primary iminium ion lead to the formation of an imine.

The relatively higher instability of enamines makes them great precursors in organic synthesis. The lone pair on the nitrogen is a resonance donor to the C=C bond making it a nucleophilic center:
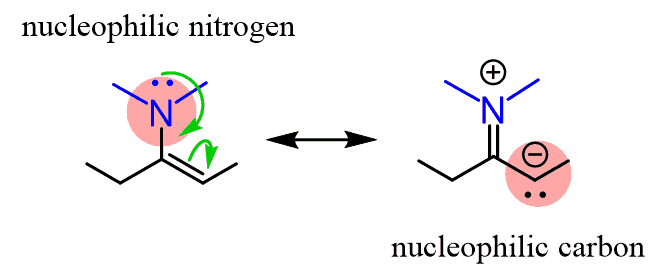
Enamines are particularly known for Stork Enamine Synthesis, which utilizes enamines as carbon nucleophiles to form new C–C bonds as a milder alternative to other condensation reactions. This is a topic for the chapter on Alpha Carbon Chemistry, so you don’t need to worry about it now.
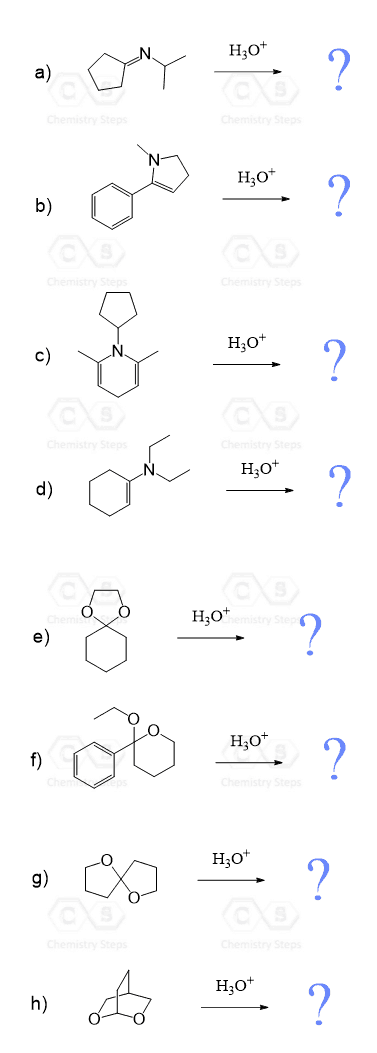
Can Water Attack the Iminium Ion?
In case you were wondering, yes, imines, and especially iminium ions are quite polar and prone to nucleophilic additions, for example, by hydride ions or organometallic nucleophiles. So, another possibility of getting rid of the positive charge on the nitrogen is the nucleophilic attack by water, pushing the reaction in the reverse direction. However, this does not lead to a significant product – it’s just a ping-pong process in the equilibrium.

Now, just because it does not lead to the formation of a new product, the nucleophilic attack of water on imines should not be ignored though. You see, if we look at it in the direction of adding water to the iminium intermediate, it will eventually break the C–N bond by restoring the carbonyl group. In other words, this is the hydrolysis of imines and enamines that leads to the formation of the corresponding aldehyde or ketone. It is all about how much water and acidity is used to control the direction of the C=N ⇌ C=O equilibrium.

We will discuss the hydrolysis of imines and enamines in the next article, so for now, let’s summarize what we learned about the formation of imines (and apparently enamines too).
Summary: Formation of Imines and Comparison with Enamines
Imines, also known as Schiff bases, are compounds containing a carbon-nitrogen double bond (C=N), formed by the nucleophilic addition of primary amines to aldehydes or ketones, followed by the elimination of water. This process occurs under mildly acidic conditions, which activate the carbonyl group toward nucleophilic attack. The mechanism involves the addition of the amine to the carbonyl carbon, proton transfers, and loss of water to form the imine.

In contrast, when secondary amines react with aldehydes or ketones, they form enamines instead of imines. This is because secondary amines lack a second N–H proton, which prevents the final step necessary to form the C=N bond. Instead, a β-hydrogen is removed, leading to the formation of a C=C double bond adjacent to the nitrogen. Both imine and enamine formation proceed through a common iminium ion intermediate, which then follows different elimination pathways depending on the type of amine.