In the previous post, we talked about the preparation of amines and one of the possible approaches was the direct SN2 reaction between an alkyl halide and a primary amine. The disadvantage of this method is the polyalkylation which occurs because the newly forming amines react with the initial alkyl halide.
Because of this, an alternative method was developed for the synthesis of primary amines.
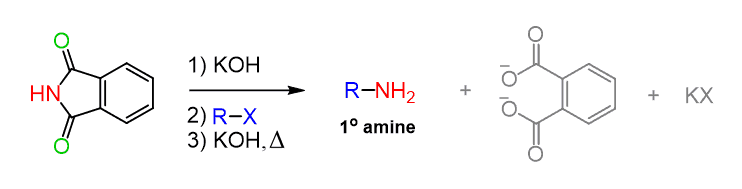
This is called the Gabriel synthesis and it relies on phthalimide as the nucleophile. The idea here is to use a bulky nitrogen which can only perform one nucleophilic substitution:
The N–H bond of the imide, being between two electron-withdrawing carbonyl groups, is quite acidic (pKa ~ 8.3). So, to convert the nitrogen into a better nucleophile, it is first deprotonated by a hydroxide or another strong enough base:
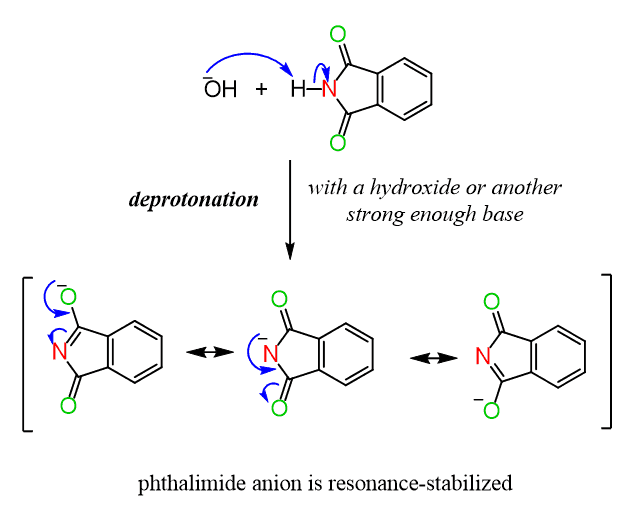
The resulting nucleophilic anion is then reacted with an unhindered methyl or primary alkyl halide in an SN2 reaction to form an N-alkylated phthalimide. The one last transformation remaining is to convert the alkylated imide into the corresponding amine and this is by hydrolyzing it much like what we have seen in the hydrolysis of amides.
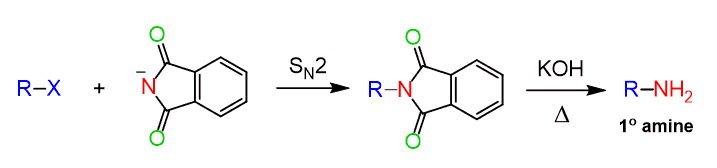
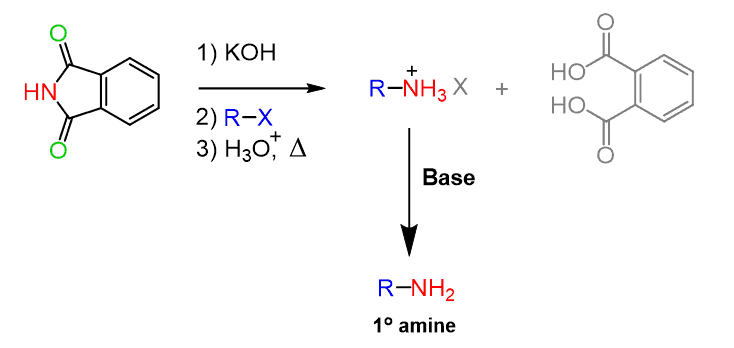
Let’s put together a summary of the Gabriel reaction for preparing1° amines in which the overall result is nucleophilic substitution of X by NH2, so the Gabriel synthesis can be used:
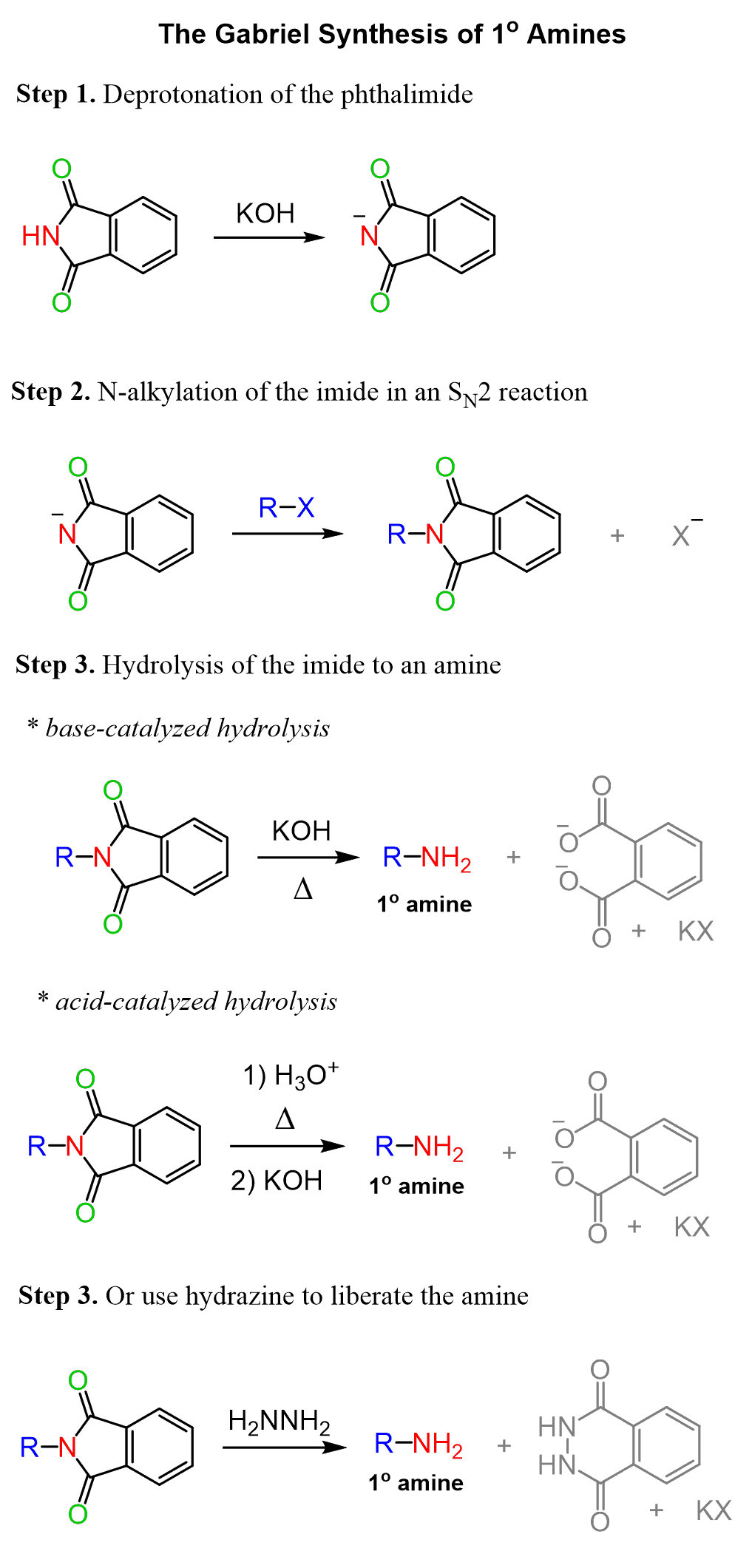
Now, the amide hydrolysis is often more efficient under acidic conditions and we may predict that the amine liberated from the imide is going to be in the protonated form since the reaction is carried on in acidic condition. This requires an extra step of treating the reaction with a base and in addition, the amide hydrolysis does require harsh conditions – remember amides are the least reactive among the carboxylic acid derivatives.
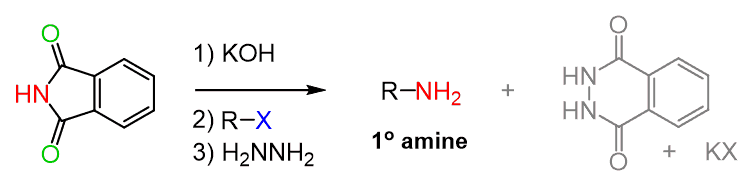
Because of this, an alternative way of getting a neutral amine is possible by reacting the alkylated imide with hydrazine which is a good nucleophile and replaces the desired amine:
Check Also
- Preparation of Amines
- The Gabriel Synthesis of Primary Amines
- The Hofmann Elimination of Amines and Alkyl Fluorides
- Imines from Aldehydes and Ketones with Primary Amines
- Enamines from Aldehydes and Ketones with Secondary Amines
- The Reaction of Amines with Nitrous Acid
- Reactions of Amines Practice Problems
