We have seen that chemically equivalent protons give one signal in NMR spectroscopy. For example,
How many signals do you expect to see in the 1H NMR of ethane?
Despite having 6 hydrogens, it only shows one signal, and this is because all the H’s are identical (chemically equivalent), they are in the same environment:
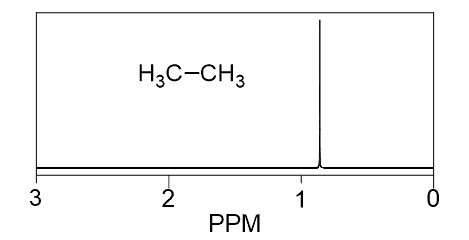
All 6 hydrogens are in the same environment – surrounded by the same types of atoms at the same distance and angle. They are chemically equivalent (react the same way) and interact with the magnetic field in the same way as well.
On the other hand, bromoethane gives two signals because there are two types of protons; the ones next to the electronegative bromine and the ones connected to the methyl carbon:

So, remember, chemically equivalent protons give one signal and chemically not equivalent protons give two signals.
There are two types of chemically equivalent protons; homotopic and enantiotopic.
Homotopic Protons
Homotopic, simply means identical. For example, all the protons in ethane are homotopic. Even though each proton is physically different, we say that they are identical because the rotation about the C-C single bond is so fast that all the protons averagely appear in the same environment.
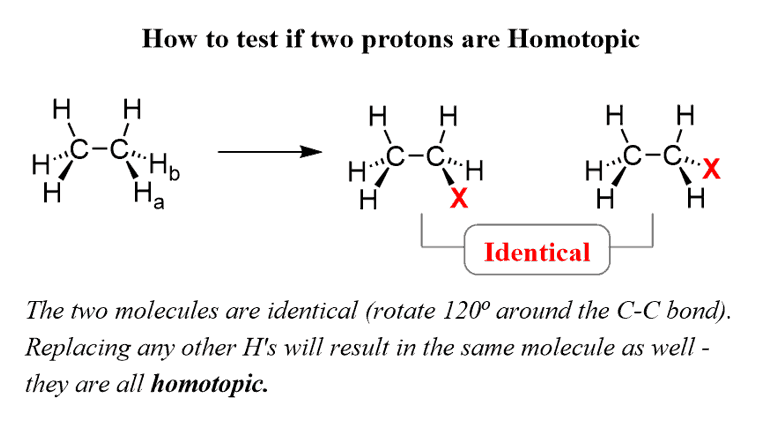
There is also an operation that can be done to check the relationship between two protons in the molecule. If replacing two protons with a different group (X) gives the same compound, the protons are called Homotopic.
If replacing two protons with a different group (X) gives a pair of enantiomers, the protons are called Enantiotopic.
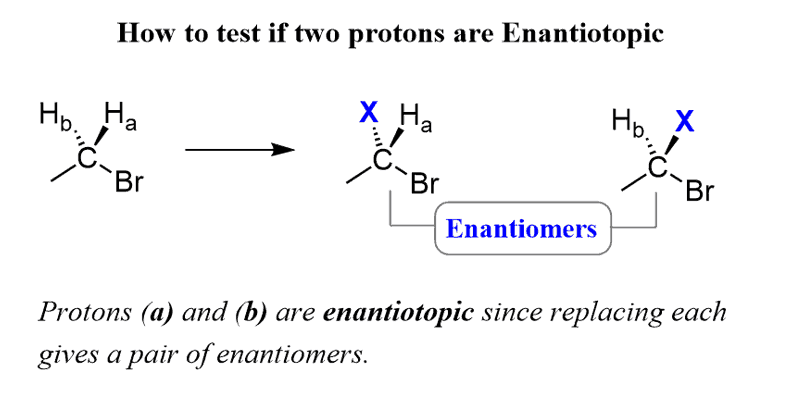
Notice that an achiral molecule became chiral and that is why enantiotopic groups are sometimes called prochiral groups.
Now, homotopic and enantiotopic (if in achiral environment) protons are chemically equivalent and give one signal:

Protons (a) and (b) are enantiotopic and give one signal.
The signals have multiple lines but each set of these lines (peaks) represents one signal. One signal can have many peaks and that is the splitting which we will discuss in the next post.
Diastereotopic Protons
The most common set of nonequivalent protons that you will hear about in your course is going to be the diastereotopic protons.
If replacing two protons with a different group (X) gives a pair of diastereomers, the protons are called Diastereotopic.
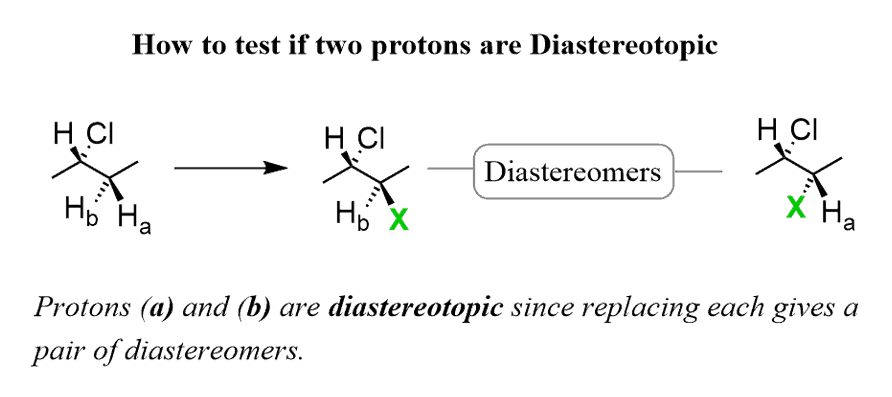
Diastereotopic protons are not chemically equivalent and give two signals:
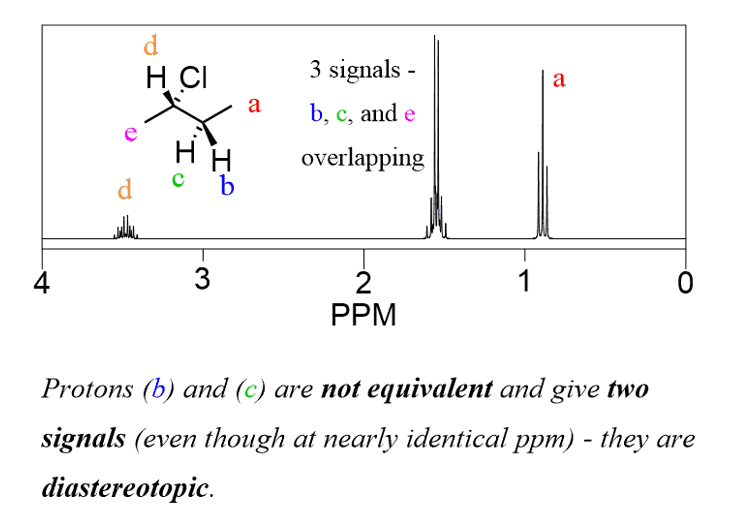
The signals of these molecules have more complex patterns but what is important for now is to emphasize that diastereotopic protons are not chemically equivalent and give two signals.
Is there an easier way than swapping atoms and looking at what molecules we get to determine if the protons are homotopic, enantiotopic, or diastereotopic?
The Shortcut to Homotopic, Enantiotopic or Diastereotopic
Replacing the hydrogens in order to determine the relationship of the protons and hence the number of NMR signals may look overwhelming and confusing and if it all seems too much for determining the relationship of the protons and the number of expected signals, here is what you need to do!
Remember, if the two protons are exchangeable through either a symmetry plane or a symmetry axis, they will give one NMR signal.
For example, the following molecules have a lot of hydrogens but because they are symmetrical, these hydrogens are in an identical environment – they have the same connectivity, they have the same neighbors and therefore are shielded and deshielded to the same extent and resonate at the same frequency:

Most of the time this will be enough to give you an idea of what to expect on the NMR spectrum of a given compound.
However, if you also need to determine specifically whether the protons are homotopic, enantiotopic, or diastereotopic, use this quick flowchart instead of the swapping method:
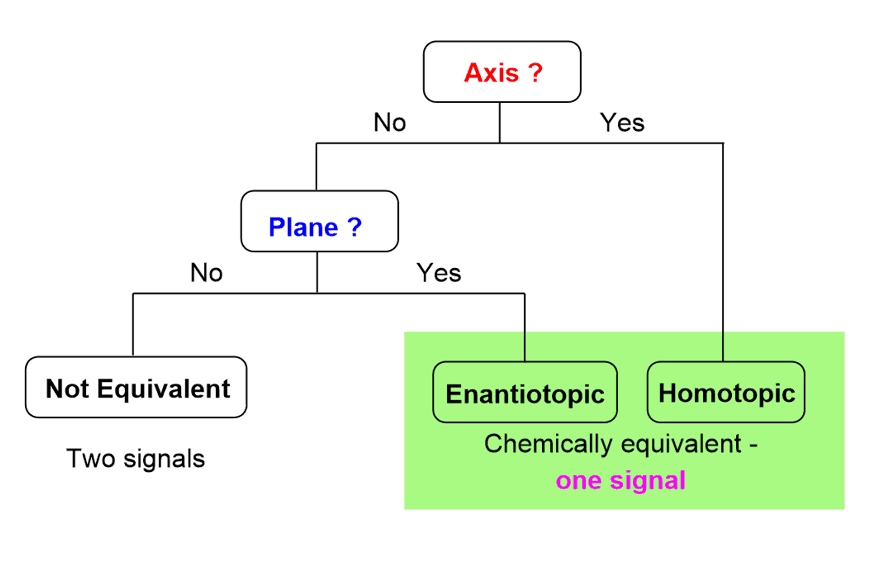
Check if the two protons are exchangeable by a symmetry axis or can be reflected through a plane of symmetry. If they have either of these, they are equivalent and give one signal. To determine the relationship of these protons, remember that the presence of a symmetry axis means they are homotopic, and if there is no axis, but there is a plane of symmetry, then the protons are enantiotopic.
If the protons are not related by these symmetry elements, they are not equivalent and will give two NMR signals.
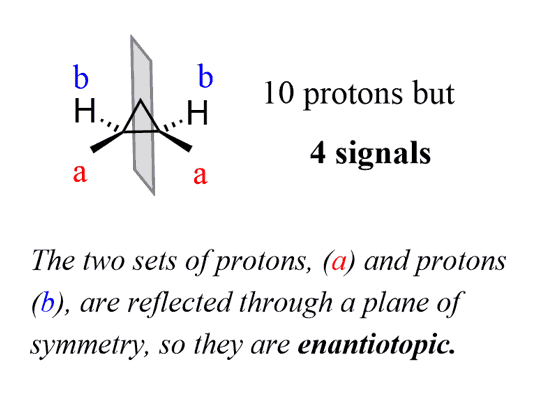
Let’s see a few examples. The two protons (a) and protons (b) in the following molecule are enantiotopic because they are reflected through a plane of symmetry:
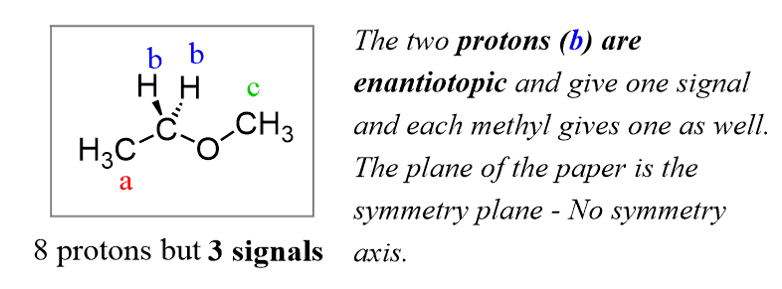
Another example, and this time, 8 protons give only three signals. Protons b are enantiotopic since they are reflected through a plane of symmetry (but no axis of symmetry in which case – homotopic) and all the protons in each methyl group are identical because of the free rotation about the single bonds:
The following two molecules do not have a plane of symmetry, however, protons (a) give one signal because they are homotopic – they are interchangeable through a symmetry axis:
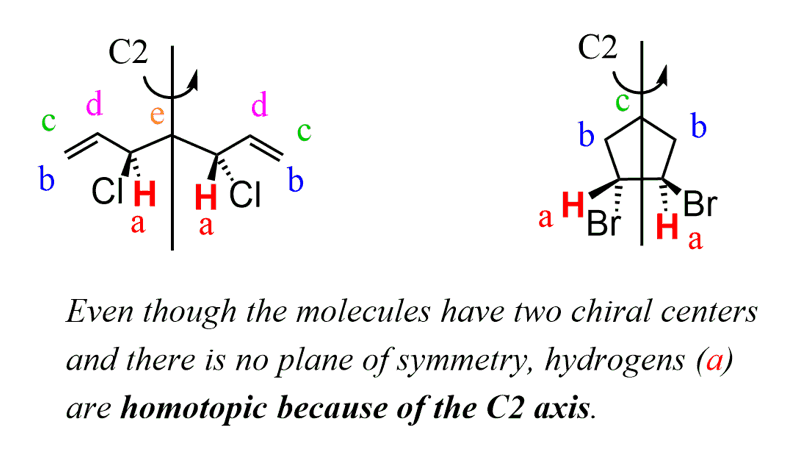
So, keep in mind, although the plane of symmetry should be the first thing (doesn’t have to be, that’s my and many other chemists’ way of doing it since it is easier to find) to check for when determining the number of signals, you should also look for a symmetry axis as both indicate chemically equivalent protons.
In the following molecules, I wanted to point out the presence of diastereotopic protons and emphasize something important!
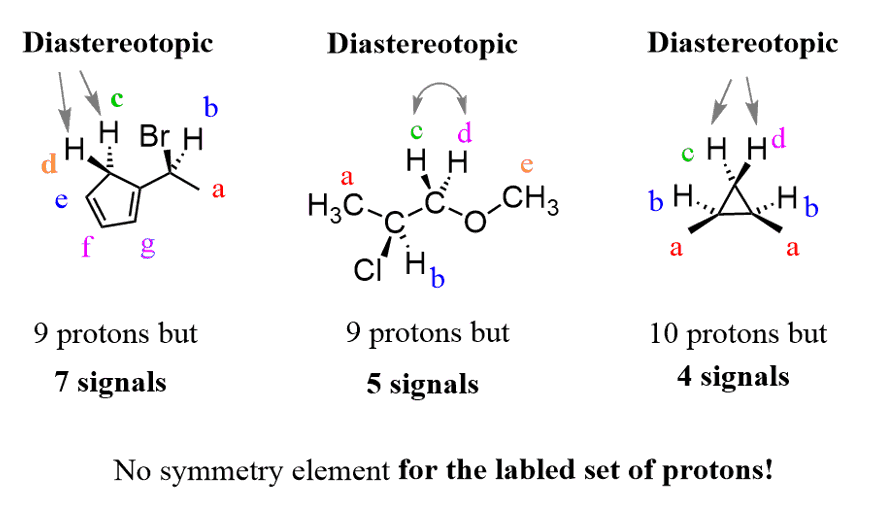
So, what is the important part I mentioned above? If we pay close attention, we can see that the last molecule (on the right) has a plane of symmetry passing perpendicular to the screen, yet we are saying that protons c and d are diastereotopic.
And yes, they are diastereotopic, because remember, the symmetry elements we are talking about are pertaining to the given pair of protons– not necessarily the entire molecule.
Heterotopic Protons
There is one more case scenario – What if neither a plane nor an axis of symmetry reflects two protons and they are not stereochemically related?
In this case, they are still chemically not equivalent but specifically, these are a pair of (constitutionally) heterotopic protons.
For example, replacing the following two protons with another group gives two constitutional isomers, which is why they are called constitutionally heterotopic or simply heterotopic protons:
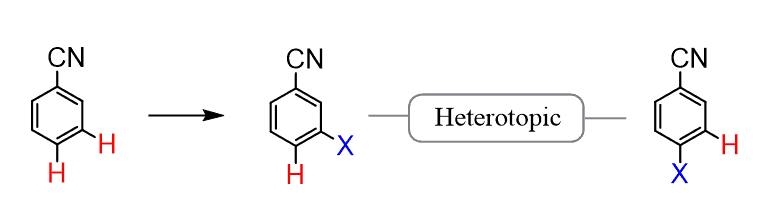
As expected, heterotopic protons give different NMR signals since they are in different environments.
You may be wondering; how come we don’t hear so much about heterotopic protons? Well, the answer is, that they are on different carbon atoms, and being a few atoms away, they don’t naturally look similar and there is no questioning whether they can give identical signals or not.
To summarize, homotopic and enantiotopic protons are chemically equivalent and give one signal. Locate them with a symmetry axis or a plane of symmetry respectively.
Diastereotopic and heterotopic protons are chemically nonequivalent and give two signals. None of these are detectable by a symmetry element. If doubting the relationship, do the swapping test.
Need some practice determining homotopic, enantiotopic, diastereotopic, and heterotopic protons? Check this article:
Homotopic Enantiotopic Diastereotopic Practice Problems
*Note: Enantiotopic hydrogens give different signals in a chiral environment. For example, when measuring the NMR in a chiral solvent.
In most cases, however, it is measured in achiral solvents, and one signal is observed for enantiotopic protons.
*Note: All the NMR spectra in the text are software-generated. This doesn’t change anything we discuss!
Check Also
- NMR spectroscopy – An Easy Introduction
- NMR Chemical Shift
- NMR Chemical Shift Range and Value Table
- NMR Number of Signals and Equivalent Protons
- Homotopic Enantiotopic Diastereotopic and Heterotopic
- Homotopic Enantiotopic Diastereotopic Practice Problems
- Integration in NMR Spectroscopy
- Splitting and Multiplicity (N+1 rule) in NMR Spectroscopy
- NMR Signal Splitting N+1 Rule Multiplicity Practice Problems
- 13C NMR NMR
- DEPT NMR: Signals and Problem Solving
- NMR Spectroscopy-Carbon-Dept-IR Practice Problems

Great i now i m clear
This is super helpful. Thank you so much!
Thanks
Clear Definition… Now I’m clear
Well explained! Thank you