Let’s start today’s discussion with simple terms: Both inductive and mesomeric (resonance) effects tell us whether one part of the molecule withdraws or donates electron density to another part of the molecule.
Now, why and how does one part of the molecule shift the electron density in the molecule? In most cases, it boils down to the electronegativity, or more accurately, to its difference between the atoms in the molecule.
The Inductive Effect
Recall from general chemistry that a polar covalent bond is formed when two atoms with different electronegativities are connected. In these bonds, the shared electron pair of the covalent bond is not in the middle of the two atoms. There is a higher density towards the more electronegative element, and as a result, both elements have partial charges. Mainly, the more electronegative atom bears a partial negative, and the other atom has a partial positive charge which are indicated by the delta plus (δ+) and delta minus (δ-) symbols:

This essentially is the inductive effect: it is an electronic effect that occurs through sigma (σ) bonds. If the atom or the group donates electron density, it is said to have a positive inductive effect (+I). Atoms or groups that pull electron density from another atom or a group through sigma bond(s), are electron-withdrawing and denoted as –I groups.
Perhaps the most important feature of the inductive effect is that it works through several sigma bonds.
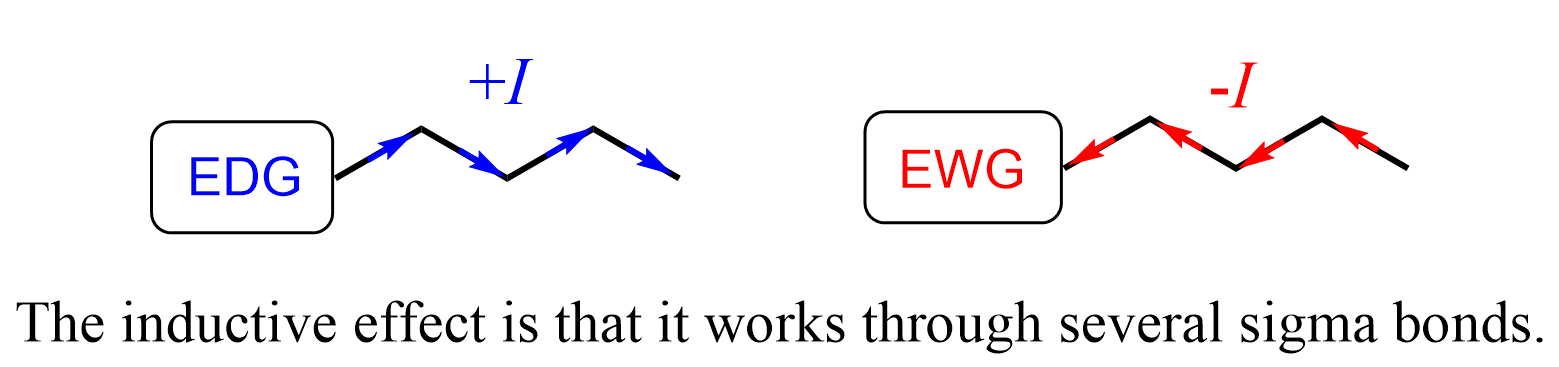
Why is this of great significance? We know that organic chemistry studies the compounds of carbon mostly connected to other nonmetals. These are covalent compounds and if you remember from resonance structures and acid-base chemistry, atoms “do not like” to be charged (recall the factors stabilizing a given resonance structure).
One of the ways the charges are stabilized is by the presence of an electron-withdrawing and electron-donating group. An anion will be stabilized by an electron-withdrawing, and a cation by an electron-donating group:

Inductive Effect and Acid Strength
Let’s see how the inductive effect influences the acidic and basic properties of molecules. For example, 2-fluoroethanol is a stronger acid than ethanol as seen from their pKa values:
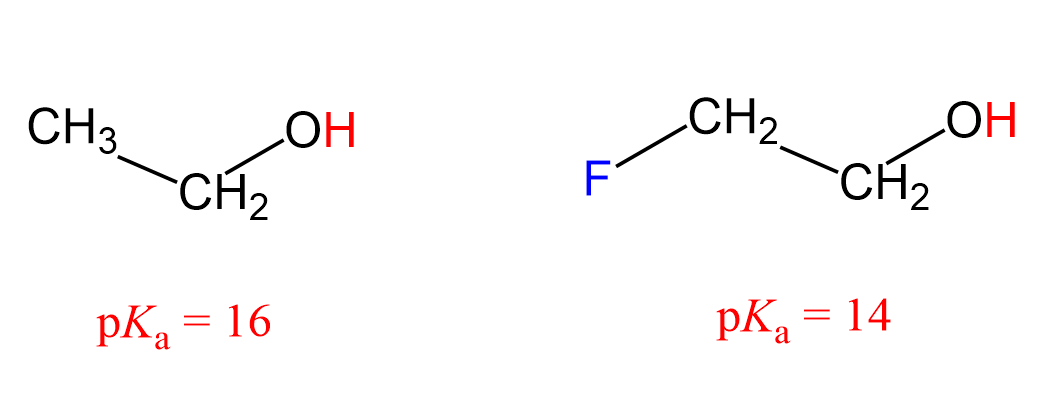
What makes 2-fluoroethanol a stronger acid? The acid strength increases with better stabilizing the negative charge of its conjugate base, so what happens here is fluorine pulls the electron density from the negatively charged oxygen thus stabilizing the conjugate base of the alcohol.
The way it works is that the F-C bond reduces the electron density of the carbon which in turn does the same to the other carbon, and finally, the carbon connected to the oxygen helps it handle the negative charge by reducing its electron density:

This pattern is very common and very well-studied for carboxylic acids. The presence of a halogen increases their acidity through the electron-withdrawing inductive effect. The greater the electronegativity of the halogen, the greater the inductive effect and thus the stronger the acid.
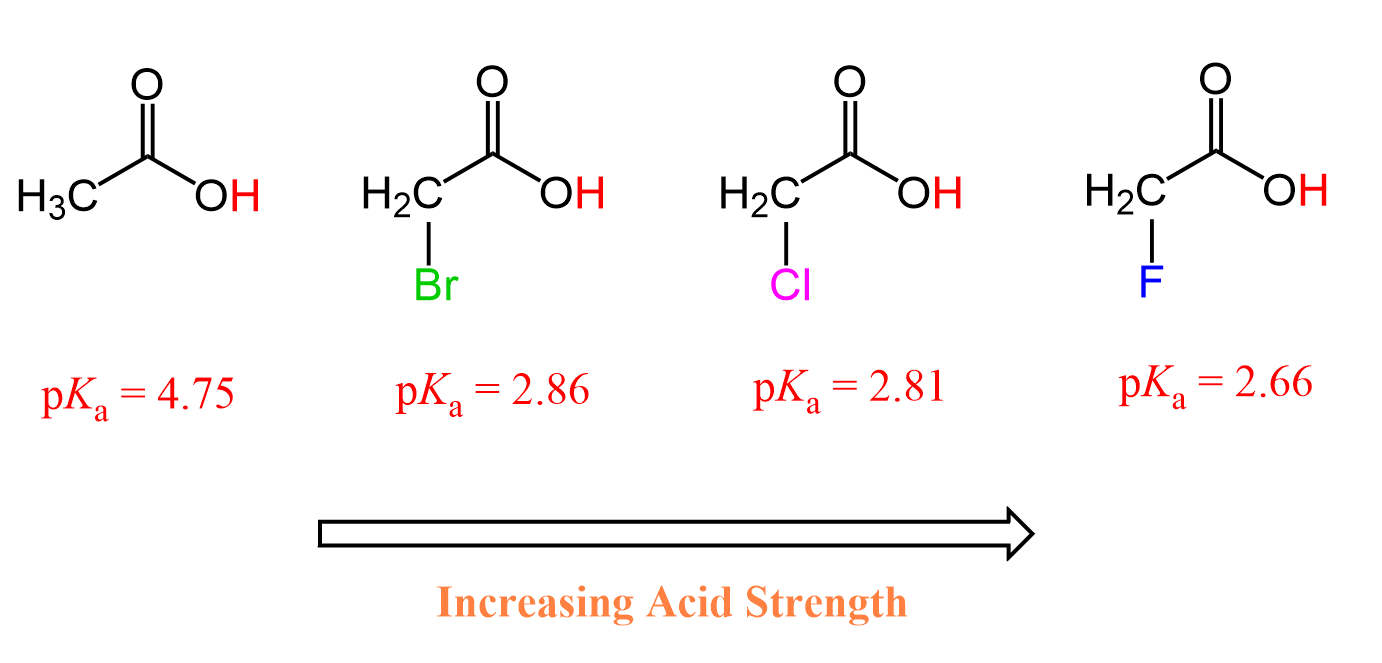
Fluoroacetic acid is stronger than chloroacetic acid which is stronger than bromoacetic acid which, in turn, is stronger than acetic acid by itself because the electronegativity of these halogens decreases in this order: F > Cl > Br.
Interestingly, it is not only the nature of the electron-withdrawing atom that affects the charge stabilization but also its distance from the carboxylate group. This is expected because the inductive effect gets weaker with the increasing number of sigma bonds between the two centers:
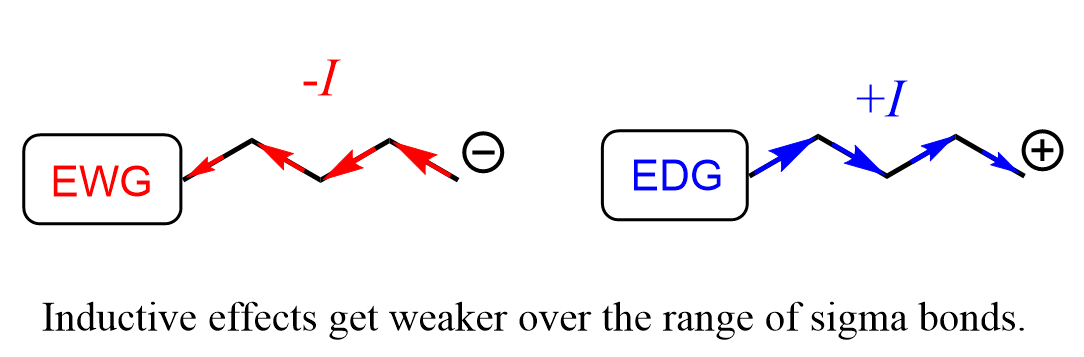
For example, compare the acidity of butanoic acid with 2, 3, and 4-chlorobuatanoic acid:

2-chlorobuatanoic acid has the lowest pKa because the chlorine is the closest to the carboxylic group and thus the inductive effect is the most profound.
Electron-Donating Inductive Effects
The classical example demonstrating the electron-donating inductive effect is the increasing stability of carbocations with the number of alkyl groups bonded to the positively charged carbon atom. This is because of the electron-donating effect of alkyl groups:
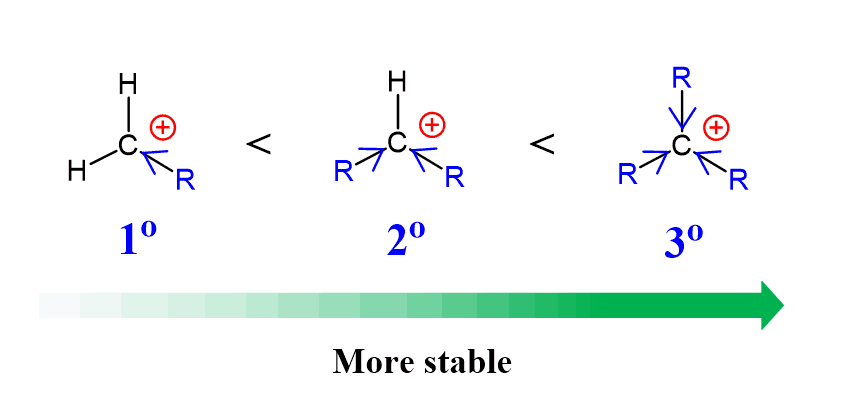
To keep this argument fair, we should also mention that the carbocation is also stabilized by what is called hyperconjugation which is the charge-stabilization by pushing some electron density of the adjacent σ bond to the empty p orbital of the carbocation:

We talk more about carbocations and hyperconjugation in SN1 and E1 reactions, so feel free to check those out as well.
Electron-Donating Groups and Acidity
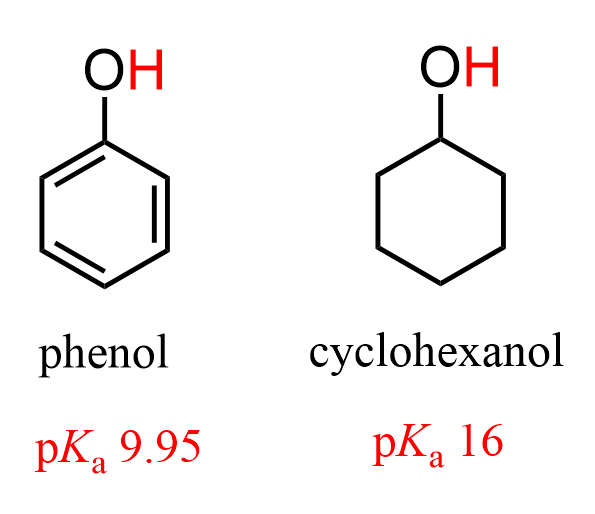
Let’s also consider how an inductive electron-donating group (+I) affects the acidity. Phenol is a very important compound, and it is very acidic compared to aliphatic alcohols because of the resonance stabilization of its conjugate base:
The acidity of phenol decreases when there is an alkyl group on the aromatic ring because alkyl groups are activators of aromatic compounds. For example, the pKa of p-cresol (4-methylphenol) is 10.26:
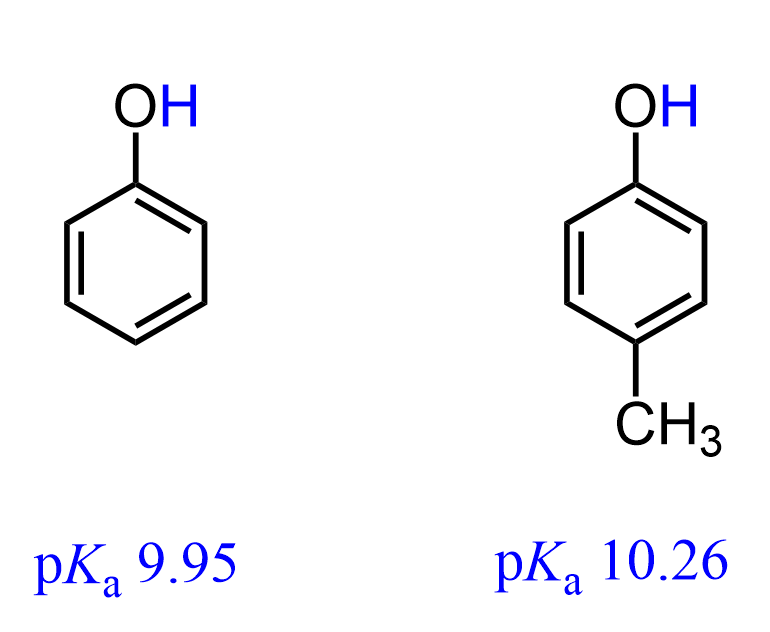
The reason for this is that once phenol dissociates (recall acid dissociation) into its conjugate base, the negative charge on the oxygen is further destabilized by the positive inductive effect of the methyl group. It donates additional electron density to the oxygen through 5 bonds, thus making it less stable, and an unstable (stronger) conjugate base means a weaker acid:

This electron donation activates the aromatic ring towards electrophilic aromatic substitutions.
Mesomeric (Resonance) Effect
Another way of shifting electron density in a molecule occurs via the resonance effect, which, because of old literature, is also known as the mesomeric effect.
The principal difference here is that the mesomeric effect works through π bonds. Remember, when discussing resonance structures, we saw that they always involve a π bond (double or triple) because we cannot break single bonds in resonance structures.
The simplest example for illustrating the mesomeric effect is the comparison of the acidity of alcohols and carboxylic acids. Carboxylic acids are a lot more acidic, even though upon dissociation, the negative charge ends up on the same element, which is oxygen:
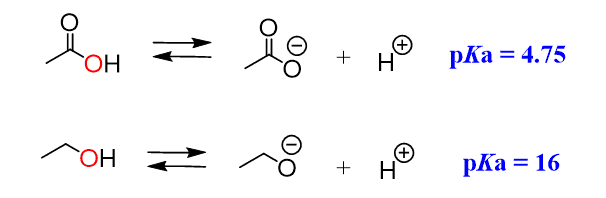
This is because the electrons on the oxygens of carboxylic acid are delocalized (in resonance with the other oxygen) and the negative charge is handled by both atoms, while the oxygen in the alcohol handles the negative charge alone:
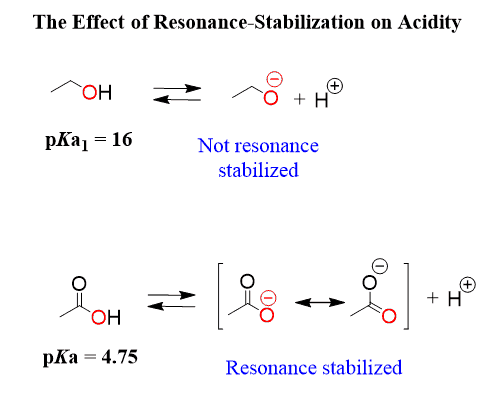
Like the inductive effect, the resonance effect is not restricted to one bond. In fact, it works in longer ranges than the inductive effect. For example, a bit earlier, we saw that the presence of an alkyl group decreases the acidity of phenol by electron-donating inductive effect. Let’s now see what happens to the pKa of phenol when there is a methoxy group on the ring:
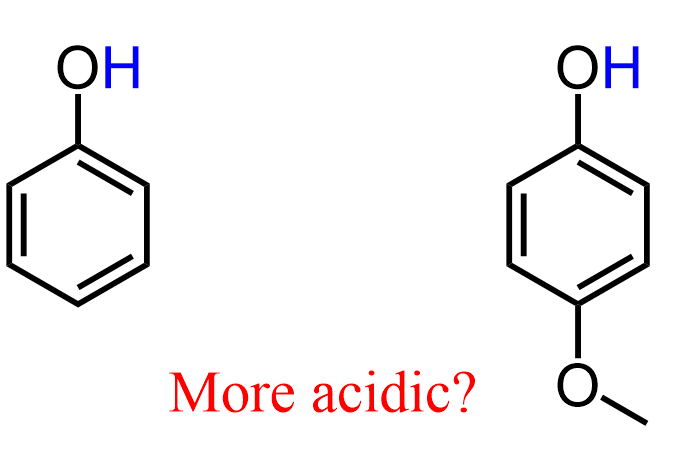
You might be thinking: Isn’t the oxygen an inductive electron-withdrawing group? Based on everything we discussed about the inductive effect, one may rightly argue that the oxygen of the methoxy group should be pulling some electron density via an inductive effect, thus stabilizing the conjugate base of 4-Methoxypheno,l which, in turn, should make the molecule more acidic:
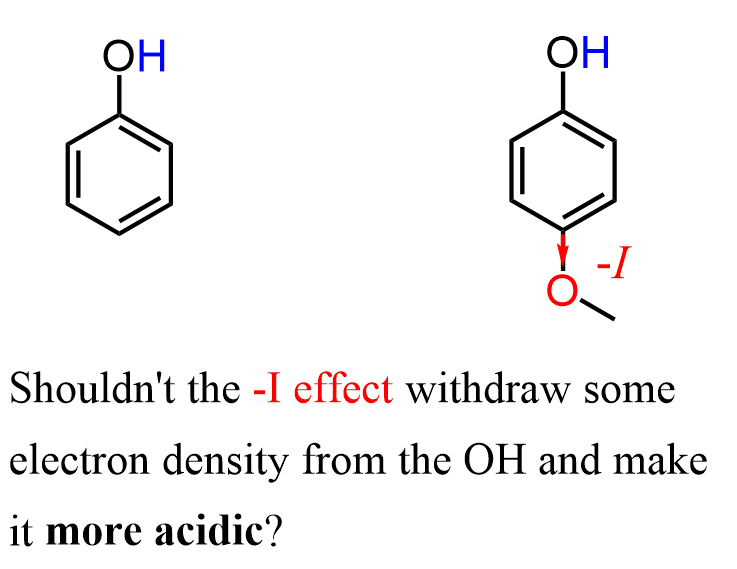
Interestingly, the pKa values suggest that the conjugate base of 4-Methoxyphenol is less stable than that of phenol.
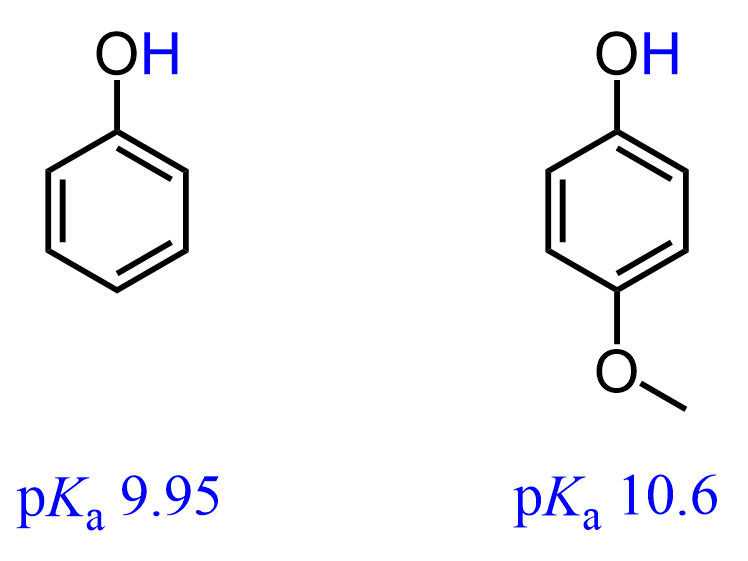
This is because one of the lone pairs on the oxygen is in resonance with the π electrons of the aromatic ring, which allows it to donate additional electron density to the negatively charged oxygen:

This is an example of a positive mesomeric effect (+M) that ranges through several π bonds.
Both effects indeed exist, and the oxygen is electron-withdrawing by inductive, and electron-donating by mesomeric effect:

What you need to remember is that the resonance effect is generally more predominant, and thus, the electron-donating effect, in this case, is what predominates. This feature is not characteristic of the methoxy group only, and we can see the resonance-donating effect of electronegative elements in many aromatic compounds such as phenol itself, aniline, and halogenated benzenes. This is a separate topic on its own, which we cover in electrophilic aromatic substitution reactions.
Electron-Withdrawing Mesomeric Effect
Contrary to the methyl and methoxy groups, which donate electron density to the aromatic ring and the oxygen connected to it, π-bond-containing groups such as nitro and different carbonyl derivatives increase the acidity of phenol. For example, both 3-nitrophenol (m-nitrophenol) and 4-nitrophenol (p-nitrophenol) are more acidic than phenol, but what is also important is that p-nitrophenol is more acidic than m-nitrophenol:
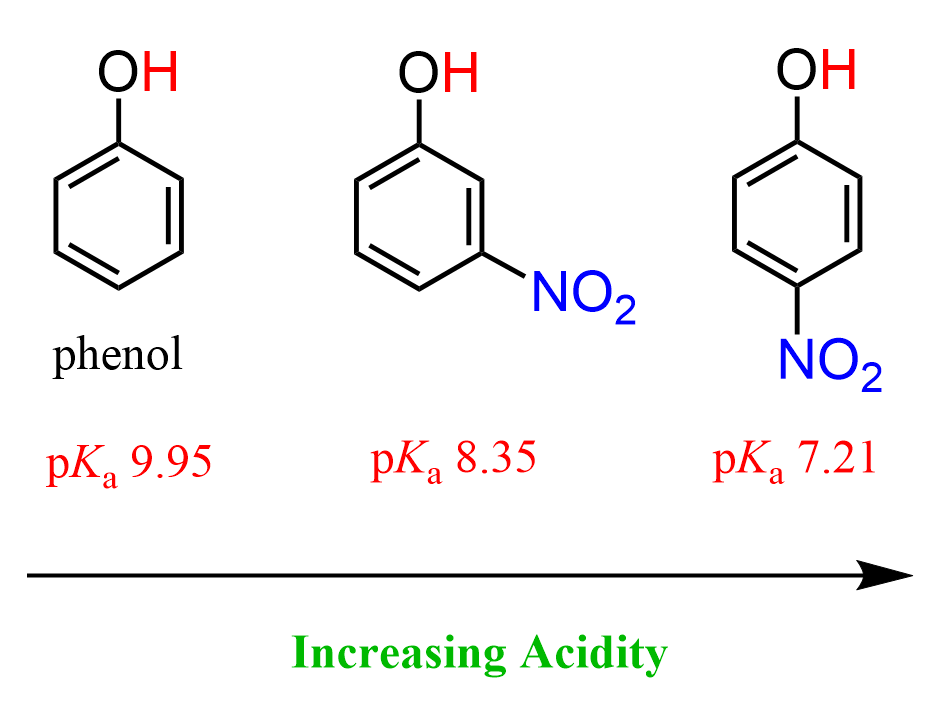
So, let’s address these observations in order. First, why does introducing a nitro group make phenol more acidic? One of the explanations is the inductive electron-withdrawing effect of it because nitrogen is more electronegative than carbon, and it pulls the electron density from the ring, which in turn helps the oxygen in handling the negative charge:

Second, there is also the mesomeric effect. If we draw the structure of the nitro group, we can see that the nitrogen is connected to one of the oxygens via a π bond which allows to draw a resonance structure where this oxygen takes the electrons of the π bond and thus in a long-range delocalizes the negative charge of the deprotonated oxygen of phenol:

Notice that when the nitro group is in the meta position, this resonance delocalization is not possible, and that is why the para-nitrophenol is more acidic than the meta isomer.
Notice also that we couldn’t explain the higher acidity of the para isomer by the inductive effect because the nitro group is closer to the OH in the meta isomer, so it should have been more acidic. This shows once again that the resonance effect is more predominant than the inductive effect.
Overall, electron-withdrawing groups deactivate aromatic compounds in electrophilic aromatic substitutions.
Another such example is aniline, where the nitrogen, without bonded oxygens, is directly connected to the aromatic ring. The difference with the nitro group is that it is electron-donating by resonance effect because of the lone pair.

Like in nitrophenol, the nitrogen in aniline is still electron-withdrawing by inductive effect. The electron-donating effect of nitrogen can be seen in many electrophilic aromatic substitutions of aniline, which are not within the scope of this article, but you can read them in the linked article.
Interestingly, if an acid is present in the solution, it protonates the nitrogen, converting the NH2 to NH3+ group, which is no longer an electron donor because there is no lone pair on the nitrogen anymore. Moreover, the nitrogen is now an even stronger electron withdrawer because it is positively charged with a stronger inductive effect:

You can read more about the resonance effect on the acidity and basicity of molecules in this post.
Inductive and Resonance (Mesomeric) Effect Strengths
Like many other characteristics, such as, for example, the acid or base strength, the electron-donating or withdrawing property is a relative measure. It depends on what other group, the one we are studying, is connected to. We can think about this in terms of electronegativity: saying an atom is electronegative is not an absolute statement because it must be in reference to another element. Same for the acidity, oxidizing-reducing power, etc. We know that halogens are often classified as electronegative elements, and this is because, compared to all the metals and the majority of nonmetals, they are more electronegative. To avoid ambiguity, for the electron-donating and withdrawing notion, the strength is generally in reference to hydrogen.
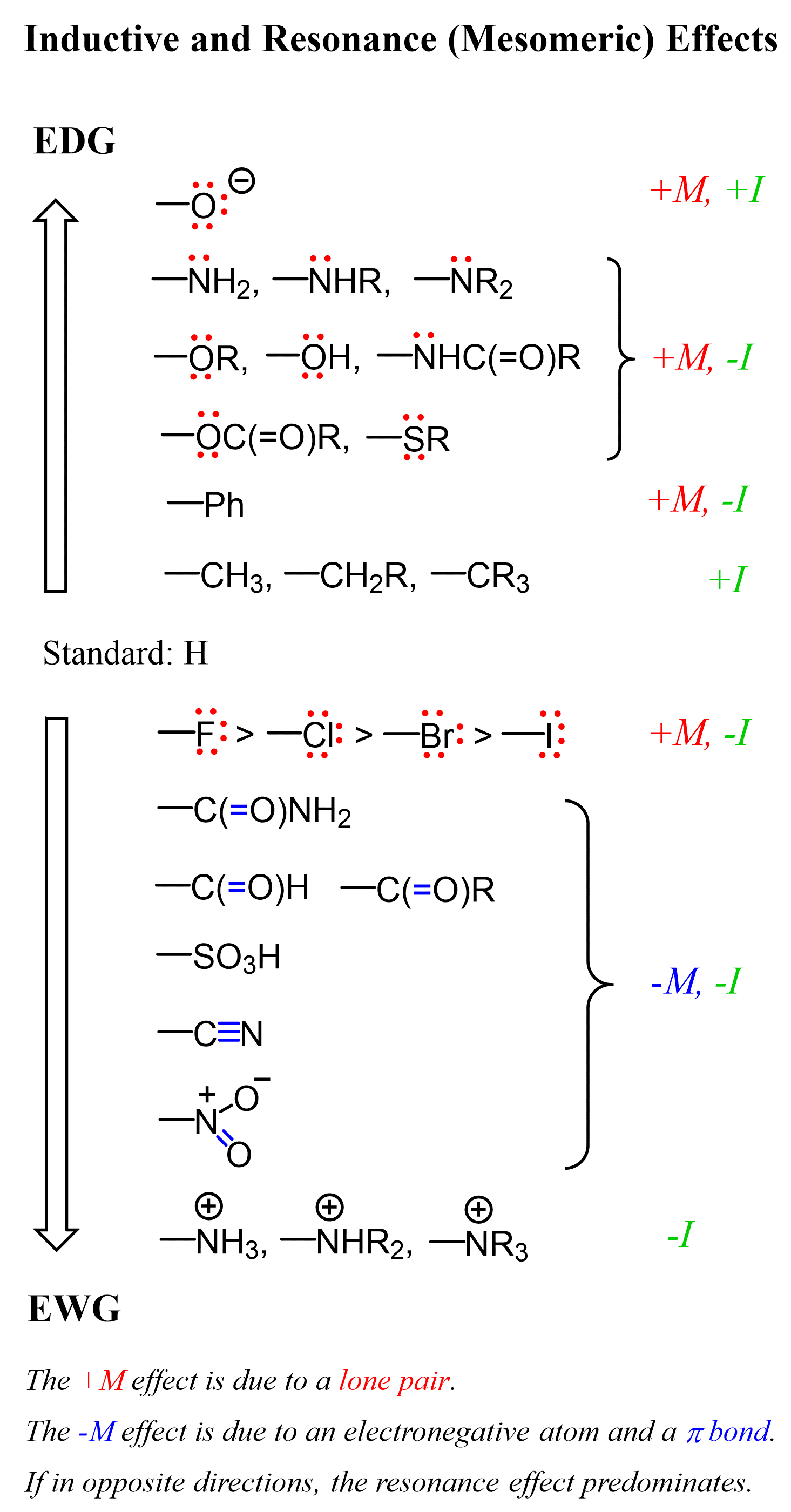
At the top of the list, we have the electron-donating groups, and notice that, except for the alkyl groups, they are all heteroatoms with a lone pair(s). The importance of the lone pair is that it allows for a resonance donation, which is generally more predominant than the inductive effect, which is why the group is electron-donating despite the atom being more electronegative than the carbon it is connected to. The phenyl group is an electron-withdrawer by the inductive effect because the carbon atoms are sp2 hybridized, and these are more electronegative than sp3 carbon. On the other hand, it is generally an electron donor by the resonance effect due to the presence of π bonds, which can delocalize electrons to stabilize a positive charge.
Halogens are inductively electron-withdrawers because they are all more electronegative than carbon. However, when connected to a π bond(s), they are also electron donors by the resonance effect, and a good example of seeing the overlap of these opposite effects is their influence on aromatic compounds in electrophilic substitutions.

You may not have covered this chapter yet, so I will just leave the link to the corresponding article here without going too much into the details.
At the bottom of the table, we have the electron-withdrawing groups. The resonance-withdrawing feature is due to the presence of an electronegative element connected to a π bond, which allows accepting the electrons via delocalization. Protonated amines are electron-withdrawing by inductive effects because nitrogen, being electronegative, is even more so once it is protonated and positively charged.
Summary of Inductive and Mesomeric Effects
Let’s summarize the key points of this discussion.
- Both inductive and mesomeric effects are responsible for some shifts in the electron density in the molecule.
- The inductive effect occurs via sigma bonds, whereas the resonance (mesomeric) effect occurs via π bonds.
- The inductive and mesomeric effects can occur via multiple bonds.
- For electron-donating groups, a positive sign is used (+I, +M), and for electron-withdrawing groups, a negative sign is used (–I, –M).
- The same group may have aligned or opposite inductive and mesomeric effects. When the effects are opposite, the resonance effect is more predominant.
- Inductive and resonance effects influence a molecule’s acidity and basicity.
- Inductive and resonance effects play an important role in activating or deactivating aromatic compounds, thereby directing the electrophile to the ortho, para, or meta positions in electrophilic aromatic substitution reactions.
Check Also
- Resonance Structures
- Activating and Deactivating Groups
- Organic Acids and Bases
- Organic Acid-Base Mechanisms
- Acid Strength and pKa
- How to Determine the Position of Equilibrium for an Acid-Base Reaction
- Factors That Determine the pKa and Acid Strength
- How Resonance Affects Acidity and Basicity
- How to Choose an Acid or a Base to Protonate or Deprotonate a Given Compound
- Lewis Acids and Bases
- Basicity of Amines
- Organic Acids and Bases Practice Problems
- Organic Acids and Bases Quiz
