The integration in NMR tells us the number of protons represented by a given signal. To be more accurate, let’s mention that it is the ratio of the protons behind each signal.
For example, we have seen that chloroethane gives two signals because the protons of the CH2 group are different from those of the CH3 group:

Now, each signal is also characterized by integration. You will see an integral sign and the corresponding number underneath. This number indicates how many protons give rise to the signal.
Let’s see how it works on the NMR spectra od chloroethane and 2-bromopropae:

The height of each integral is proportional to the area of the given signal and the area is determined based in the number of absorbing protons.
The integral of signal b is 1.5 times taller than the one for signal a since the proton ratio is 3 : 2. On the second spectrum, the integral of signal a is six times taller than signal b since the ratio here is 6 : 1.
Notice that we are not talking about the height of the signal as it may not necessarily represent the number of protons. For example, OH or NH peaks are most often broad and short – shorter than the proton ratio.
So, remember, the number of protons is represented by the area of the peak and not the height.
What if the Integration Doesn’t match the Formula?
NMR instruments don’t know what we are trying to do – all they do for integration is measure the relative intensity and give them to us with some convenient numbers. These numbers may not match the actual number of protons as it is only their ratio.
For example, a compound with a molecular formula C7H12 gives the following integration:
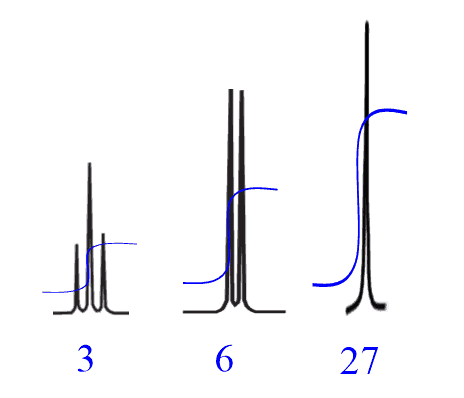
This cannot be the number of protons since there are, in total, 12 protons based on the chemical formula. So, in these situations, you need to divide the numbers by the smallest integration value and check if the sum of new values matches the chemical formula:

Integral values 1, 2 and 9 satisfy the chemical formula, therefore, you can manually set the first signal to 1 and the rest will adjust automatically.
It may also not be a set of perfect whole numbers and sometimes you need to adjust the signal parameters if still in the lab. In any case, keep in mind that integration numbers may not be exact, and it is okay to go based on some rounding. For example, the ratio 1.1 : 8.95 : 12.05 is reasonable to consider as 1 : 9 : 12.
If the numbers are fractions, they also need to be simplified. For example, having a 1 : 1.5 ratio likely indicates a 2 : 3 ratio since we cannot have 1.5 protons.
The 2:3 ration often indicates an ethyl group:

A 1 : 1.5 ratio can also indicate a 4 and 6 protons per signal based on the molecular formula. For example, the C6H10 molecular formula cannot have 2 and 3 protons since the sum must be 10 protons, therefore the number of protons in this case would have to be 4:6.

The correlated ratio of integral heights was especially handy in the early days of NMR when a ruler was used to measure the heights and thus the number of protons:

Modern software do this calculation for us and we get the ratio of protons automatically.
Integration of the OH peak
Integration of exchangeable protons such as the OH peak can be less than one and often the OH peak may not be present on the spectrum at all.

So, do not rely on and set the OH or NH peak as 1 when adjusting the integration. Find the C-H peak with the lowest integration value.
This should sum up the essentials of integration in MNR spectroscopy. Ready to do some problem-solving? Check this post, there are quite a few to work on:
NMR Spectroscopy-Carbon-Dept-IR Practice Problems
Check Also
- NMR spectroscopy – An Easy Introduction
- NMR Chemical Shift
- NMR Chemical Shift Range and Value Table
- NMR Number of Signals and Equivalent Protons
- Homotopic Enantiotopic Diastereotopic and Heterotopic
- Homotopic Enantiotopic Diastereotopic Practice Problems
- Integration in NMR Spectroscopy
- Splitting and Multiplicity (N+1 rule) in NMR Spectroscopy
- NMR Signal Splitting N+1 Rule Multiplicity Practice Problems
- 13C NMR NMR
- DEPT NMR: Signals and Problem Solving
- NMR Spectroscopy-Carbon-Dept-IR Practice Problems

that’s great
Hello,
Thank you so much for nice explanation.
I tried to find the chemical structure for C7H12 formula which could match the H-NMR spectrum you suggest in section ‘What if the Integration Doesn’t match Formula?’
But I could not. It seems it should be C6H12 to match the H-NMR.
Best regards
Mohammad
Hello Mohammad,
That example was for a hypothetical molecule, and I did not think of an actual molecule that would have the given combination of splitting and integration. It was only to mention that the integration values are not always whole numbers, but they always (except for OH and other acidic protons) show the correct ratio of the protons.