We have seen, in different reactions such as the aldol condensation, alkylation, and alpha halogenation, that enolates are very good nucleophiles capable of attacking electrophilic centers.
Interestingly, the halogenation of aldehydes and ketones at the alpha position works with both acid or a base catalysis. The base catalyst converts them into enolates while the acid catalysis forms enols which are still quite good of nucleophiles.
For example, diethyl ketone can be brominated at the alpha position when threated with bromine in acidic media. The ketone is first converted into an enol which further reacts with the halogen via SN2 mechanism:
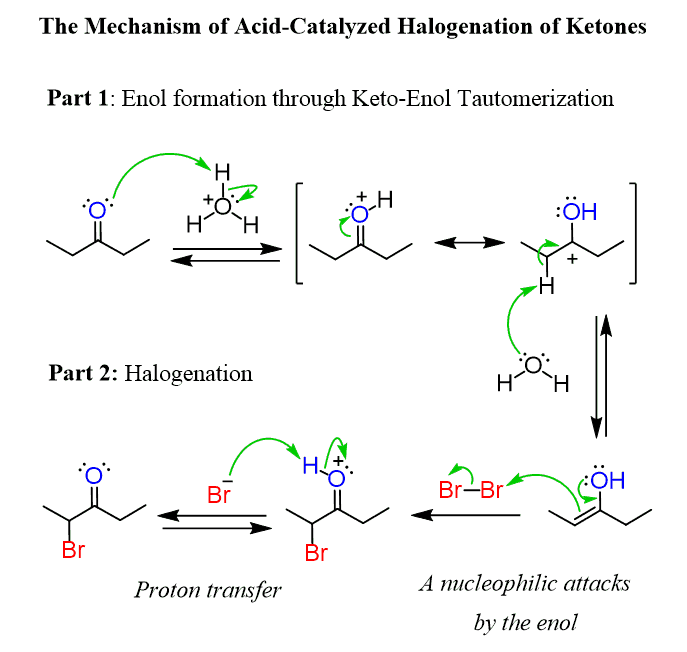
Now, what is the Mannich reaction and how is it related to enols?
Just like the example above, the Mannich reaction also involves an enol reacting with an electrophile.
So, what is the electrophile in the Mannich reaction?
Remember, earlier in the aldehydes and ketones chapter, we discussed about imines? These are obtained when aldehydes and ketones are reacted with amines.
For example, when formaldehyde is reacted with dimethyl amine, an iminium ion is formed:
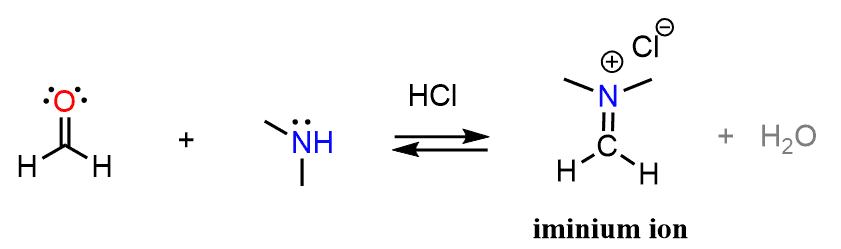
The iminium ion is quite electron deficient because of the positively charged nitrogen and therefore, it is a good electrophile. If an enol and the iminium ion were present in the same solution, we’d expect a nucleophilic attack by the enol on the carbon pushing the π bond electrons to the nitrogen and thus restoring its normal valency of three bonds and a lone pair:
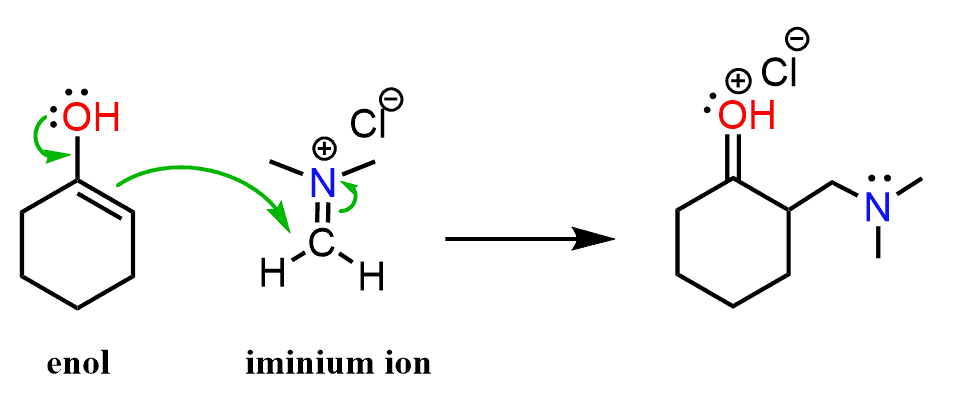
And this, essentially, is the Mannich reaction – it is a condensation of an aldehyde or ketone in form of an enol with an iminium ion producing β-aminoalkyl carbonyl compounds.
The iminium ion is most often prepared from formaldehyde although it can be any nonenolizable, that is lacking alpha hydrogens, aldehyde, ketone, or oether C-H acid such as a nitrile or nitro compound.
The imine can be generated in situ under acidic conditions and therefore, the overall reaction can be presented as follows:
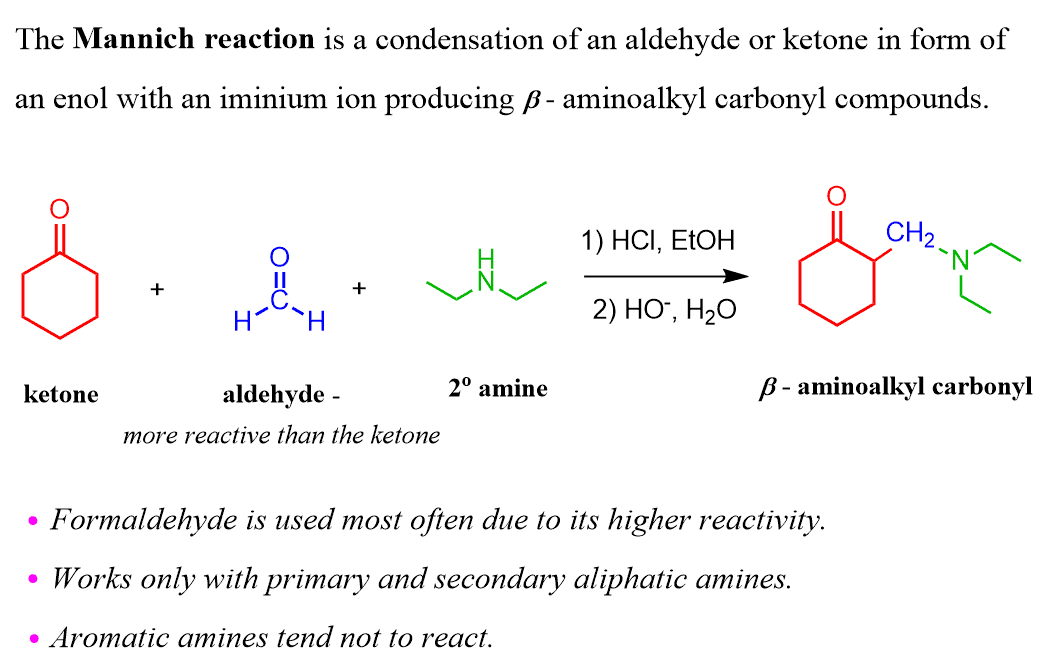
The final product contains a free base nitrogen and therefore is also referred to as “Mannich base”. The basic work up in the end is necessary to deprotonate the nitrogen in the intermediate called “Salt of Mannich base”.
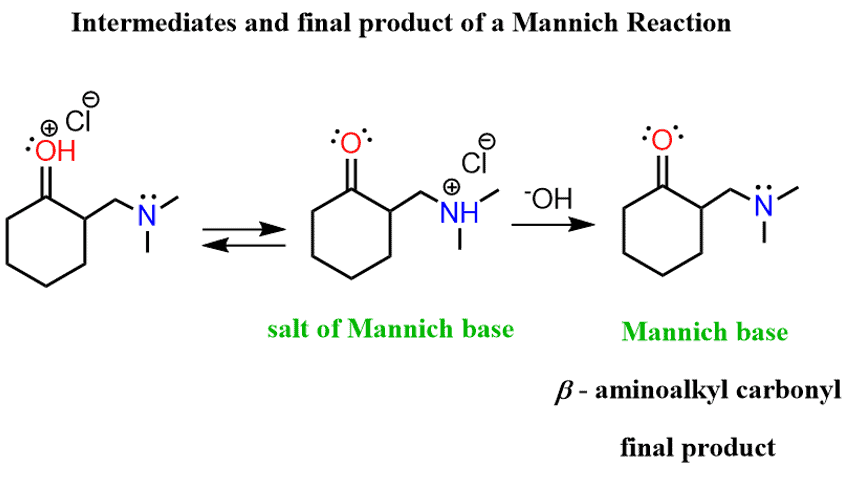
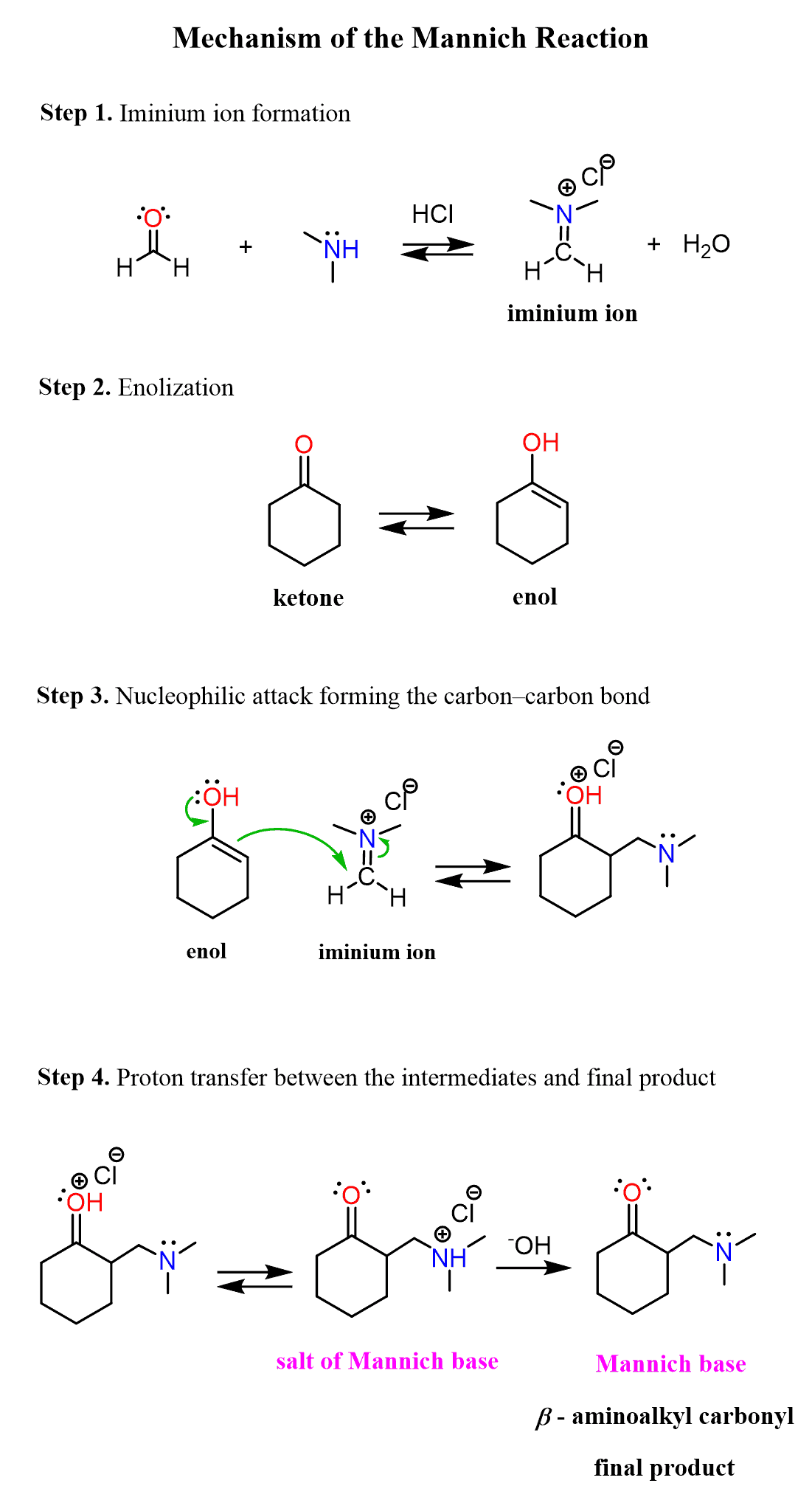
Let’s put all the steps together to build a few steps mechanism for the Mannich reaction:
If we summarize the transformation, we can say that a Mannich reaction puts a CH2NRR group on the ɑ position of a compound with an acidic ɑ carbon.
Now, let’s go over the reacting species. The ketone shown as an example in the diagram can be any active hydrogen containing compound such as an aldehyde, ester, nitroalkanes and etc.
The preference for formaldehyde is given due to its relatively higher reactivity. So, it can, in theory, be anything more reactive than the carbonyl shown before it otherwise the latter may react with the amine.
As for the amines, the Mannich reactions works only with primary and secondary aliphatic amines since aromatic amines tend not to react.
Side Products of the Mannich Reaction
Because the product of the reaction is a nitrogen base, it may react with formaldehyde producing another iminium ion which further condense with one or two additional molecules of the ketone or the aldehyde:
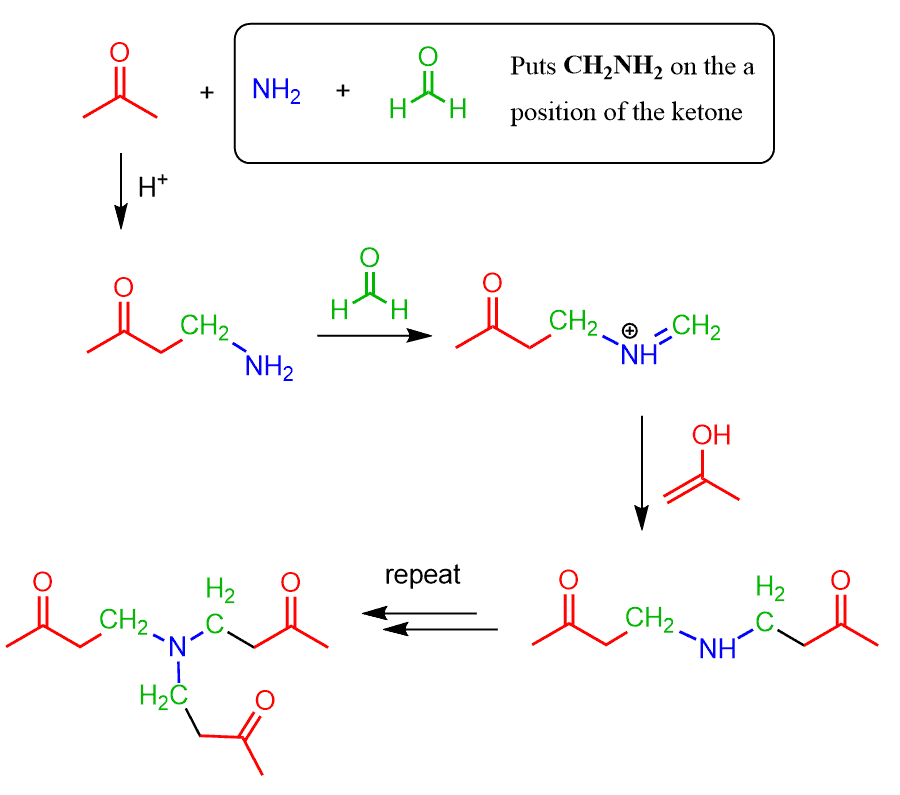
Notice that if the product of the Mannich reaction (Mannich base) was a tertiary amine, this further condensation wouldn’t occur, so this particular risk (or possibility) is only present if ammonia or a primary amine was initially used.
Another possible side product can be if the carbonyl compound has two or three acidic hydrogens. In this case, the Mannich base may condense with one or two additional molecules of aldehyde:
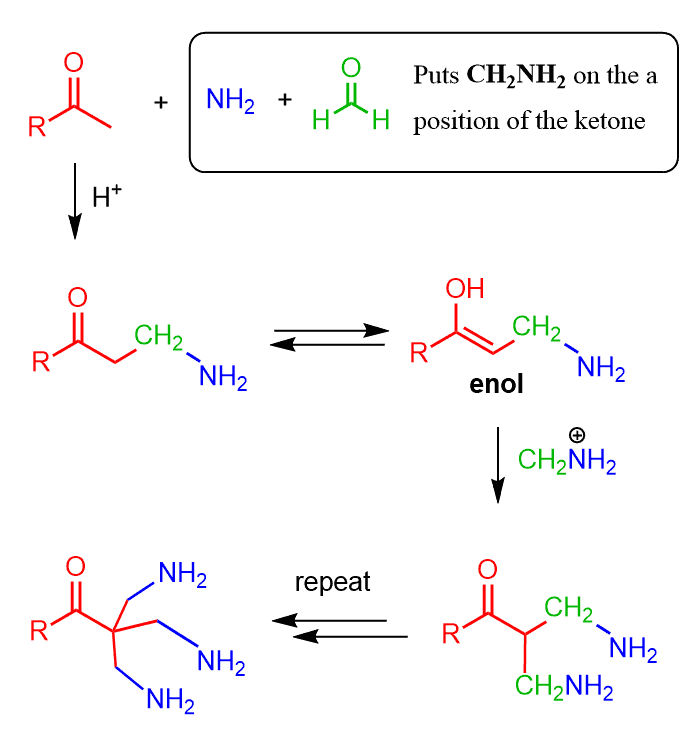
This is all I wanted to share with you about the Mannich reaction. If you need further reading, I would suggest reading it in March’s Advanced Organic Chemistry.
Check Also
- Alpha Halogenation of Enols and Enolates
- The Haloform and Iodoform Reactions
- Alpha Halogenation of Carboxylic Acids
- Alpha Halogenation of Enols and Enolates Practice Problems
- Aldol Reaction – Principles and Mechanism
- Aldol Condensation – Dehydration of Aldol Addition Product
- Intramolecular Aldol Reactions
- Aldol Addition and Condensation Reactions – Practice Problems
- Crossed Aldol And Directed Aldol Reactions
- Crossed Aldol Condensation Practice Problems
- The Cannizzaro reaction
- Alkylation of Enolates Alpha Position
- Enolate Alkylation Practice Problems
- Acetoacetic Ester Synthesis
- Acetoacetic Ester Enolates Practice Problems
- Malonic Ester Synthesis
- Michael Reaction: The Conjugate Addition of Enolates
- Robinson Annulation, Shortcut, and Retrosynthesis
- Claisen Condensation
- Dieckmann condensation – An Intramolecular Claisen Reaction
- Crossed Claisen and Claisen Variation Reactions
- Claisen Condensation Practice Problems
- Stork Enamine Synthesis
- The Mannich Reaction
- Enolates in Organic Synthesis – a Comprehensive Practice Problem
