Remember when discussing the substitution reactions, we said that the hydroxide ion (–OH) is a poor leaving group since it is quite a strong base. Therefore, the –OH cannot be expelled by a direct nucleophilic attack in an SN2 or SN1 reaction:
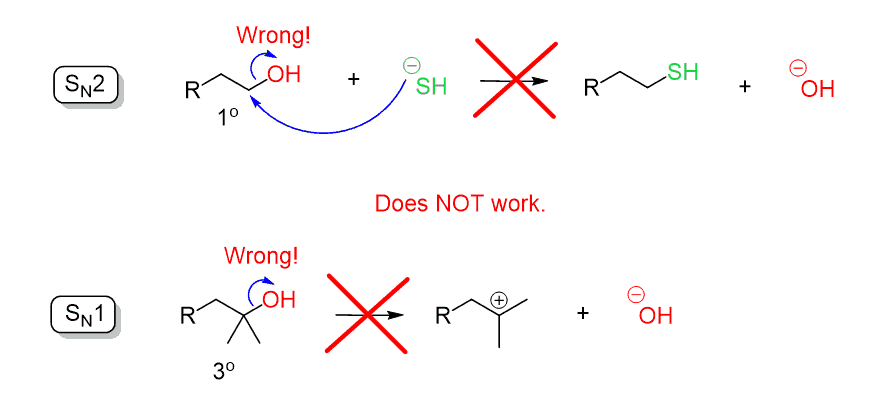
So, to perform a substitution reaction on an alcohol, the hydroxyl group must first be converted into a good leaving group. This can be, for example, a halogen that leaves as a halide ion in substitution reactions.
One way of converting alcohols in substitution reactions to alkyl halides is by reacting them with strong acids such as HCl, HBr, and HI, or using thionyl chloride (SOCl2) or phosphorus tribromide (PBr3). In both approaches, the principle behind this transformation was the conversion of the OH into a good leaving group.

In addition to these methods, the OH can also be converted into a good leaving group by reacting with sulfonyl chlorides such as p-Toluenesulfonyl chloride (TsCl), Methanesulfonyl chloride (MsCl), and Trifluoromethanesulfonyl chloride (TfCl):

The resulting products are called Tosylates (-OTs), Mesylates (-OMs), and Triflates (-OTf,) all of which are excellent leaving groups and can be used in substitution and elimination reactions:

Now, the question is why do we need to complicate our lives and deal with tosylates and mesylates if the reaction with acids works just fine, right?
Well, in general, the use of alkyl sulfonates is a better alternative to HX acids for controlling the stereochemistry of the reaction and also avoiding the use of strong acids.
So what exactly is beneficial for the stereochemistry of the reaction?
Remember, one problem with using the acids is the possible SN1 mechanism, which forms a carbocation and can lead to loss of stereochemistry and rearrangements for certain alcohols:

Unlike alcohols, mesylates and tosylates are always reacted with a salt such as NaCl or NaBr and not the acids. The use of salts is to ensure the halogens are in a form of good nucleophiles as they are not suppressed by the protons, and the reaction goes by an SN2 mechanism. No need to use an acid since the OH is already a good leaving which is another advantage because the reaction is carried out at milder conditions.
It is also worth mentioning that mesylates and tosylates are not used only for the halogenation of alcohols. They simply convert the OH into a good leaving group, after which they can participate in any substitution and elimination reaction:
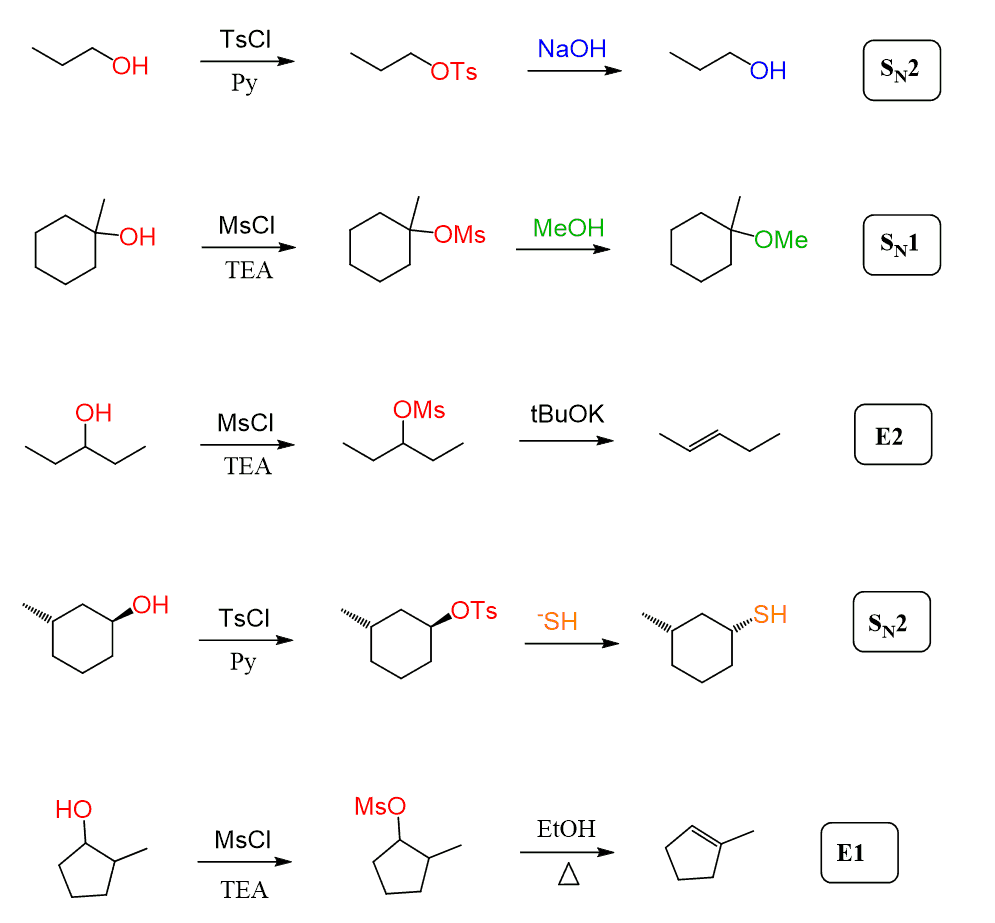
Alright, we have discussed, at this point, the idea of using these reagents, but how do they work, what is the mechanism of mesylation and tosylation?
Let’s address this one by one:
What makes Tosylates and Mesylates Good Leaving Groups
Let’s compare two reactions with a good nucleophile:

How come the first reaction does not work while the second one works very nicely? Why is the tosylate a better leaving group than the OH?
If you recalled the resonance stabilization, well done – that’s what it is. While the oxygen of the OH group bears the full negative charge on its own, the sulfonate ion has three oxygens to handle the negative charge, which is better than the only one in the hydroxyl group:

The Mechanism of Mesylation and Tosylation
Let’s discuss the mechanism for converting (R)-2-Butanol to a tosylate followed by a substitution reaction via the SN2 mechanism.
In the first step, the alcohol acts as a nucleophile, attacking the sulfur to replace the chloride.
Notice that the carbon with the stereogenic center is not involved in this step, and its configuration is still retained:

The pyridine is added as a base to deprotonate the intermediate and speed up the process of forming the Toluenesulfonate ester (tosylate).
After this step, the OH is now turned into a good leaving group, which can be kicked out by a nucleophile:

Another way of achieving an inversion of configuration via the SN2 mechanism, where the alcohol serves as an electrophile, is the Mitsunobu reaction:
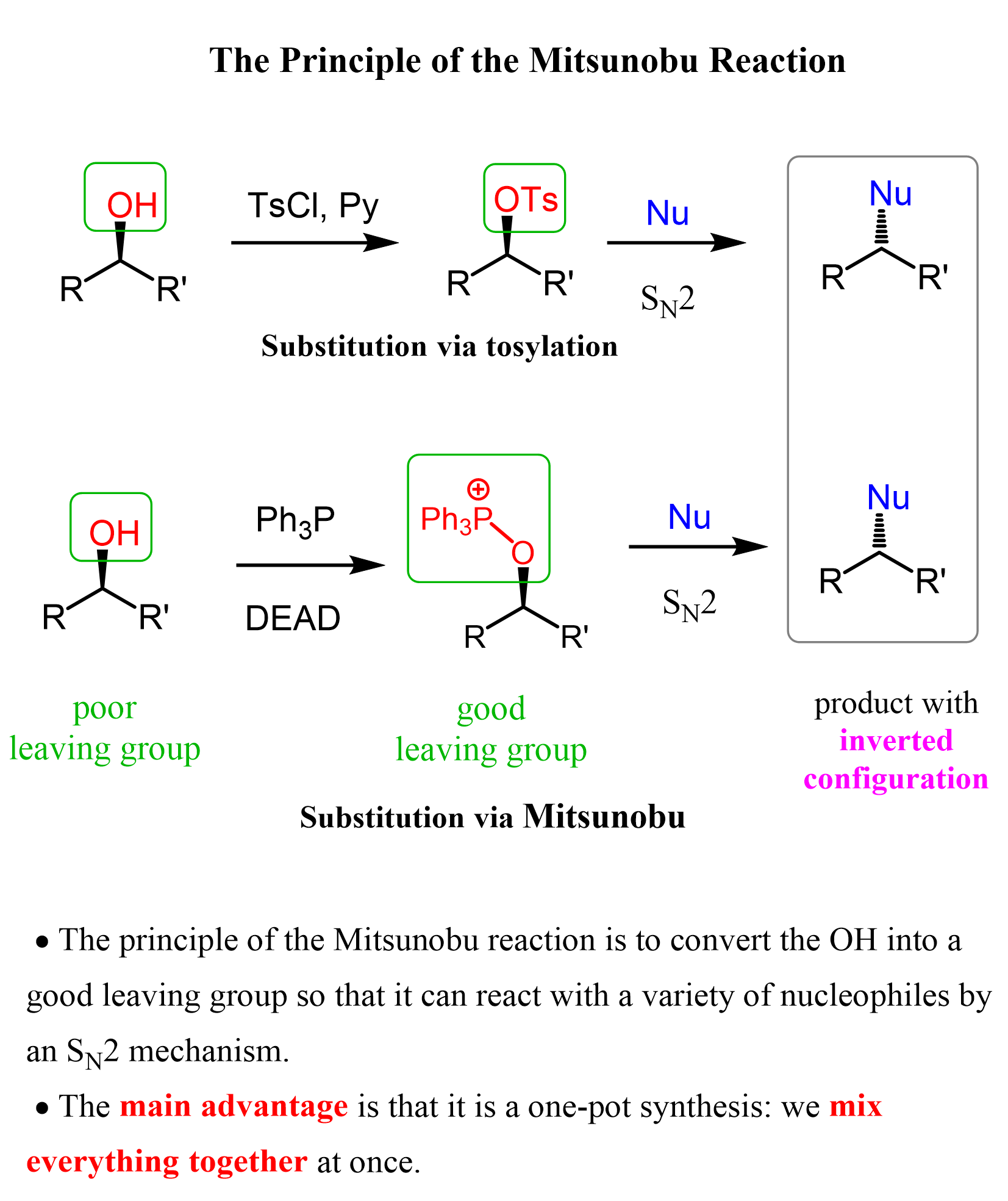
We have this covered in a separate article here, so feel free to check that as well.
Mesylation goes by a slightly different mechanism. The first step here is the deprotonation of the acidic ɑ proton by the base from methanesulfonyl chloride, which forms a sulfene. The sulfene is very electrophilic and reacts quickly with the alcohol:

These reactions will often be shown with the mechanism we saw for the tosylation in undergraduate courses.
Ask your instructor if the same mechanism as for tosylation is acceptable for mesylation.
The formation of the reactive sulfene intermediate gives a slight advantage to mesylate when working with tertiary alcohols since they react very slowly with TsCl.
The advantage of tosylation is that it is a larger molecule and turns some liquid alcohol into solids, which sometimes are preferred since they are easier to handle. Also, the aromatic ring of the tosylates allows for better visualization on a TLC plate.
But once again, the fact that it is larger makes its preparation a bit slower, and it is not as reactive in substitution reactions either.
Triflate ion is also an excellent leaving group; however, it is not covered so much in undergraduate textbooks, therefore, we will not go too much into details about the corresponding mechanisms.
However, conceptually, there is no difference – it will only be abbreviated as Tf instead of Ts or Ms, and you can simply treat it as a good leaving group when working on an exercise.
Mesylates and Tosylates in SN1 Reactions
In all the examples above, we have seen that tosylates and mesylates always react with strong nucleophiles when converted to alkyl halides, and in these cases, the SN2 mechanism will predominate.
However, mesylates and tosylates can also undergo SN1 and E1 elimination reactions when a tertiary alcohol or a weak nucleophile is used:

In general, these do not affect the principles of determining whether the reaction goes through an SN1, SN2, E1, or E2 mechanism.
Check out this 65-question, Multiple-Choice Quiz with a 3-hour Video Solution covering Nucleophilic Substitution and Elimination Reactions:
Nucleophilic Substitution and Elimination Practice Quiz


