Michael reactions are conjugate-addition reactions of doubly stabilized enolates such as malonic ester, acetoacetic ester, and the like with ɑ, β-unsaturated carbonyl compounds:

So, the Michael reaction is a particular type of conjugate addition reaction that ɑ, β-unsaturated carbonyl compounds undergo with nucleophiles.
In general, ɑ, β-unsaturated carbonyl compounds can undergo a 1,2- or 1,4-addition reaction. 1,2-addition reactions are all of those where the nucleophile attacks the carbonyl group. For example, the reaction of carbonyl compounds with a Grignard or organolithium reagent is 1,2-addition:

Both the Grignard and especially organolithiums are very strong bases, and stronger bases tend to give 1,2 carbonyl addition reactions while weaker bases give 1,4 conjugate addition. The basicity and nucleophilicity are mostly parallel for this concept.
So, unlike the organometallics, relatively weak nucleophiles such as halides, cyanide ion, thiols, alcohol, and amines, perform 1,4-addition:

One notable exception in organometallics is the reaction of organocuprates which are not as strong and therefore, they occur in 1,4-fashion:

💥 Check out this comprehensive review of the reaction of Grignard (RMgX), Organolithium (RLi), and Gilman (R2CuLi) Reagents to understand how they differ in their addition to carbonyl compounds and other electrophilic species.
Now, let’s go back to the Michael reaction. The terms Michael donor and Michael acceptor are used for the nucleophile and the electrophile, respectively.
As mentioned in the first paragraph, the most common nucleophiles in Michael’s reactions are doubly stabilized enolates. The enolates with one carbonyl group are not efficient Michael donors since they are quite strong bases.
Synthetic Strategies in Michel Reactions
This indicates that sometimes, you may need to choose between two possible paths for designing a synthesis using a Michel reaction. For example, both reactions below should yield the same product, but which one would work better?
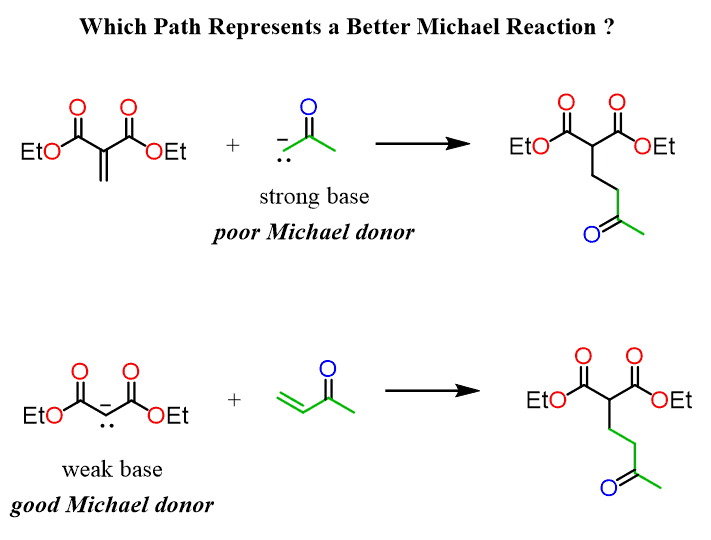
Both are Michael reactions since it is a nucleophilic addition to an ɑ, β-unsaturated carbonyl compound. However, the first option is using a regular enolate which is a strong base and not a good Michael donor.
Therefore, to obtain better yields, the second reaction involving malonic ester as a nucleophile is a better choice.
A nice approach for converting aldehydes and ketones to Michael donors is the Stork enamine synthesis and we will talk about that reaction too.

The Mechanism of Michael Reaction
Let’s first understand why the 1,4-additions occur in the first place. If we draw the resonance structures of a simple ɑ, β-unsaturated carbonyl compound, we see that there are two electrophilic positions:

These are the sites that nucleophiles can attack. For the Michael reaction, the nucleophile is generated by the deprotonation of the most acidic proton which is between the two electron-withdrawing groups:
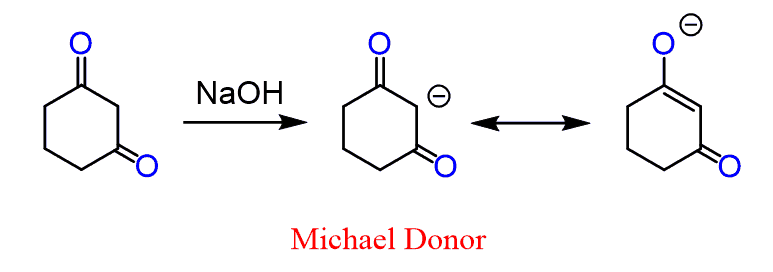
The above-shown example is a 1,3-diketone but other electron-withdrawing groups such as esters, nitriles, and nitro compounds in the same arrangement can also be Michael donors. Similarly, the electron-withdrawing group of the ɑ, β-unsaturated compound can be any of these as well.
In the next step, the nucleophilic attack on the C=C double bond generates an enolate which after the protonation tautomerizes to the corresponding carbonyl compound:

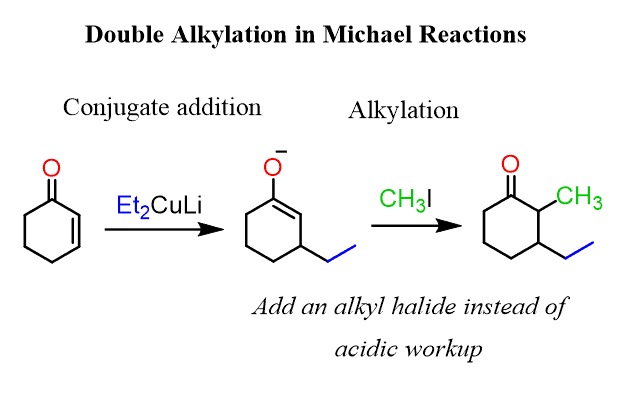
How to Achieve a Double Alkylation in the Michael Reaction
You may encounter a problem asking you to put two alkyl groups in a Michel acceptor:
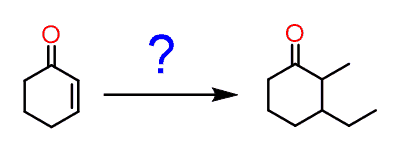
And this may look unclear since you know that using a Gilman reagent would alkylate the β position giving a carbonyl which is difficult to selectively alkylate on the target position:
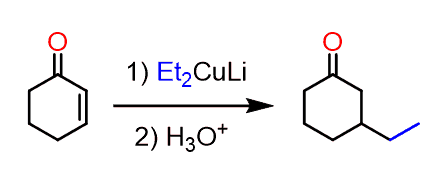
None of the sides is less substituted to make the use of LDA or NaH beneficial for a regioselective alkylation.
So, the trick here is to add an alkyl halide before the acidic workup. In this case, the intermediate enolate is still reactive and the allylation occurs on the side where the organocuprate had reacted:
In the next post, we will talk about the Robinson annulation, which is a useful variation of the Michael addition, commonly used in organic synthesis to synthesize a ring through an aldol condensation of the Michael addition product.

Need some additional practice problems? Check this comprehensive set of alpha carbon chemistry questions: