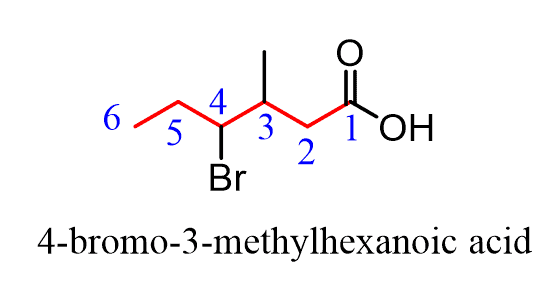
In the IUPAC nomenclature, carboxylic acids are named by adding a suffix to the parent name of the longest chain. If the parent chain is noncyclic, you need to first find the longest carbon chain containing the -COOH group and change the suffix from “ane” to “oic acid” dropping the “e” and the locant “1” in the final name:

Everything else is based on the IUPAC nomenclature rules for simple alkanes.
The substituents are numbered based on the position of the COOH group and placed in alphabetical order:
Naming Carboxylic Acids on a Ring
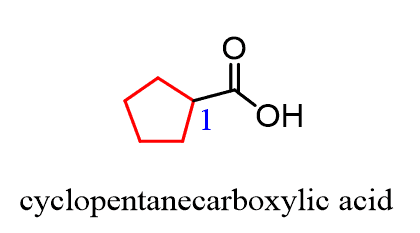
The only out-of-normal situation you may encounter is when the -COOH group is on a ring. In this case, we name the ring and add the words “carboxylic acid”:
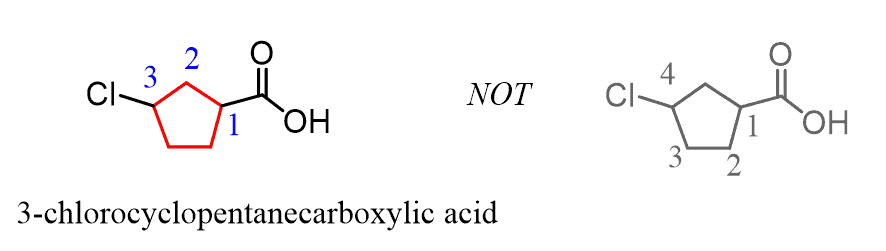
If substituents are also present, the numbering starts from the carbon connected to the COOH group and goes in the direction that minimizes the numbering of the substituents:
Naming Carboxylic Acids with Functional Groups
Carboxylic acids have higher priority than all the other functional groups and therefore, they define the parent chain and give the corresponding suffix to the compound’s name. All the other groups standing below in the functional group priority table are added as a prefix.
Here is a table of functional group priorities for reference and you can read more about naming compounds with multiple functional groups here:

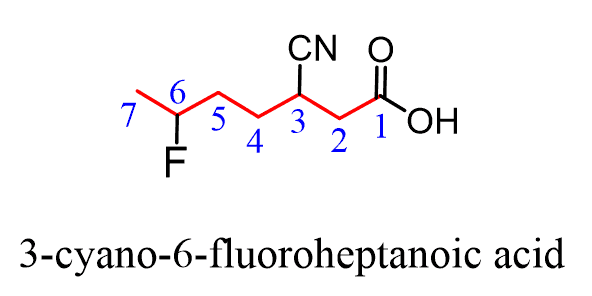
As an example, let’s name this compound containing a carboxylic acid, a halide, and nitrile groups:
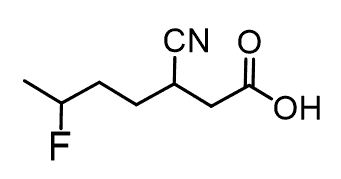
The parent chain is heptane and we have a heptanoic acid. Halogens are one of the groups that are not considered in the priority list of functional groups, so they are always substituents and get a prefix. So, in the final name, we will simply place “fluoro” in the alphabetical order.
The nitrile group has a lower priority and will get the prefix “cyano” since it is also treated as a substituent:
Common Names of Carboxylic Acids
Carboxylic acids can also be identified by their common names. These are very common, and it would be beneficial to memorize them:

When substituted carboxylic acids are named by common names, the carbon positions are often designated with Greek letters. The carbon next to the COOH is called the ɑ carbon, followed by β, γ (gamma), δ (delta), etc. The last carbon can be referred to as W (omega) positions.
For example:
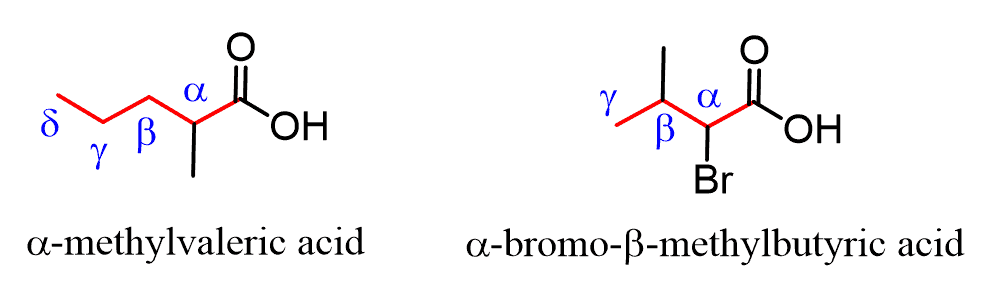
Naming Salts of Carboxylic Acids
Carboxylic acid salts are named by replacing the suffix “ic acid” or “oic acid” with “ate”. For example:

Below are some practice examples for naming carboxylic acids and their different derivatives.
Check Also
- Preparation of Carboxylic Acids
- Fischer Esterification
- Ester Hydrolysis by Acid and Base-Catalyzed Hydrolysis
- What is Transesterification?
- Esters Reaction with Amines – The Aminolysis Mechanism
- Ester Reactions Summary and Practice Problems
- Preparation of Acyl (Acid) Chlorides (ROCl)
- Reactions of Acid Chlorides (ROCl) with Nucleophiles
- Reaction of Acyl Chlorides with Grignard and Gilman (Organocuprate) Reagents
- Reduction of Acyl Chlorides by LiAlH4, NaBH4, and LiAl(OtBu)3H
- Preparation and Reaction Mechanism of Carboxylic Anhydrides
- Amides – Structure and Reactivity
- Amides Hydrolysis: Acid and Base-Catalyzed Mechanism
- Amide Dehydration Mechanism by SOCl2, POCl3, and P2O5
- Amide Reduction Mechanism by LiAlH4
- Amides Preparation and Reactions Summary
- Amides from Carboxylic Acids-DCC and EDC Coupling
- The Mechanism of Nitrile Hydrolysis To Carboxylic Acid
- Nitrile Reduction Mechanism with LiAlH4 and DIBAL to Amine or Aldehyde
- The Mechanism of Grignard and Organolithium Reactions with Nitriles
- Carboxylic Acids and Their Derivatives Practice Problems


I love how you break this down, I’m in intro to biochemistry. I have Zero chemistry experience. I wish I could chat with you to explain stuff to me. I did purchase your charts. Amazing !!
Thanks, Shannon. Great to know it was helpful.
It’s understandable. Am studying applied chemistry and I find it difficult to name compounds