Amines
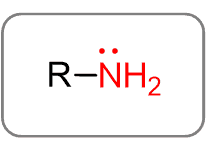
Before going into the details of naming amines, let’s first recall what they are and how they are classified.
Amines are the derivatives of ammonia (remember NH3 from General Chemistry). Replacing one hydrogen of ammonia with an alkyl group forms an amine with a general formula of R-NH2:

Depending on the number of alkyl groups connected to the nitrogen, we have primary, secondary, and tertiary amines:

We have a comprehensive article on the introduction to amines, their structure, properties, and important applications in medicine, so feel free to check it out too.
Naming Primary Amines
In general, amines can be named either by systematic or common names.
Naming amines by the systematic nomenclature follows the same rules we discussed earlier for the IUPAC nomenclature rules for alkanes.
This is the brief summary of naming a primary amine:
Step 1. Identify the longest carbon chain bonded to the amine nitrogen.
Step 2. Identify the substituents.
Step 3. Number the parent chain, giving the amine the lowest locant
Step 4. Put everything together, having the substituents in alphabetical order.
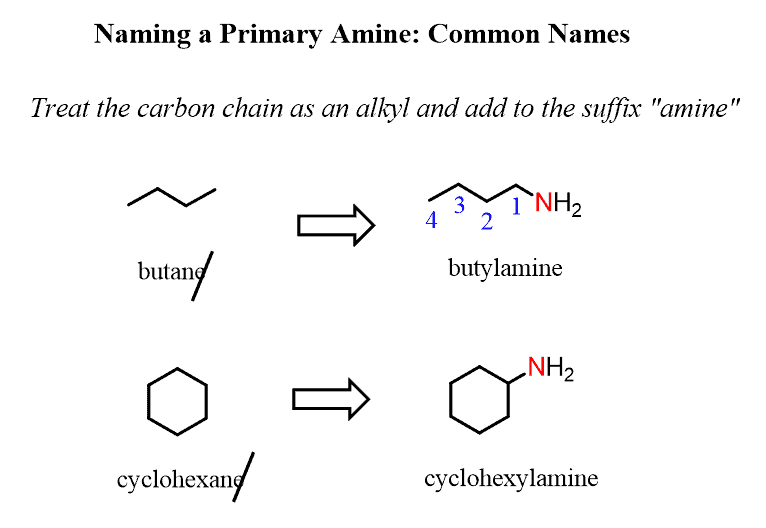
For example, butane changes to butan-1-amine, cyclohexane to cyclohexanamine:

In common names, we treat the carbon chain as an alkyl group bonded to the nitrogen atom. The alkyl group is added to the suffix “amine” forming a single word:
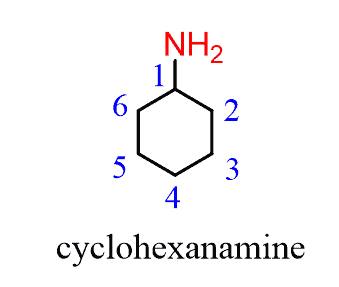
Notice that when the amine is connected to a ring, we start numbering from the carbon connected to the NH2 group. This rule always puts the NH2 group at C1, therefore, the “1” is usually omitted from the name:
When other groups are present on the ring, it is numbered clockwise or counterclockwise, depending on which direction gives the next substituent the lower number:

Let’s consider a few more examples demonstrating the priority of the amino group over other functional groups such as alkyls, halides, and multiple bonds. The list of functional group priorities can be found in this post:
How to Name a Compound with Multiple Functional Groups
The parent chain is chosen such that it is the longest carbon chain containing the carbon atom connected to the NH2 group, even if there is a longer chain without the NH2 group:

The amino group has a higher priority than alkyl groups and halides, and therefore, changes the numbering of the parent chain:

If the NH2 group is connected to a chiral center, you will also need to include the absolute configuration at the beginning of the name. Also, if there is a double, the E and Z configuration should be addressed when applicable:

Naming a Compound Where the Amino Group Is Not the Highest Priority
When the compound contains a functional group(s) that have higher priority than the amino group, then it is represented by a prefix “amino”.
For example, if we put an alcohol and an amine on the periphery of a carbon chain, the alcohol gets the priority; therefore, it is assigned with a suffix, while the amine is assigned a prefix (like the alkyl substitutes). This also indicates that we need to start numbering the carbon chain from the OH group:

So, remember the suffix and prefix of the amino group: “amine” and “amino” respectively. And for the alcohol, it is “hydroxy” and “ol”. Again, the full list of this can be found here.
Naming Secondary and Tertiary Amines
There are two case-scenarios here: all the alkyl groups connected to the nitrogen are the same, and not all the alkyl groups are the same.
When the alkyl groups are identical, they are listed with a prefix “di” or “tri” and the compound is named just like what we have seen in the common names. We name them like alkyl amines:

If the secondary or a tertiary amine has more than one type of alkyl group, then it is named as a primary amine. The parent chain is the longest chain bonded to the amine, and the other groups are named as substituents connected to the nitrogen and preceded by an “N” (in italics). This emphasizes that they are bonded to the nitrogen rather than to a carbon:

Notice, from the last two examples, that the substituents are listed in alphabetical order regardless of whether they are connected to the nitrogen or to the parent chain. I.e., the methyl groups are listed after the Br even though they are connected to the nitrogen while the Br is on the carbon chain.
And, in the last example, the order of alkyl groups goes from nitrogen (ethyl), parent chain (methyl), and nitrogen (propyl), which shows once again that we are prioritizing them in alphabetical order.
Aromatic and Other Common Amines
There are lots of amines that are simply referred to by their common names. Most of these are used as organic bases and constitute a part of biologically active and essential compounds such as amino acids, nitrogen-containing pain killers (alkaloids), as well as synthetic medicines.

Check Also
- Introduction to Amines
- Preparation of Amines
- The Gabriel Synthesis of Primary Amines
- Imines from Aldehydes and Ketones with Primary Amines
- Enamines from Aldehydes and Ketones with Secondary Amines
- The Hofmann Elimination of Amines and Alkyl Fluorides
- The Reaction of Amines with Nitrous Acid
- Reactions of Amines Practice Problems
- The Cope elimination
- Basicity of Amines
- Formation and Reactions of Imines and Enamines
- Reductive Amination
- Boc Protecting Group for Amines

Shouldn’t the molecule “2-aminohexan-3-ol” under the section “Naming A Compound Where the Amino group is Not the Highest Priority” be numbered in the opposite direction? Because the alcohol is the higher priority?
This is such an excellent question that brings up some tricky IUPAC stuff. Thank you for asking it.
The priority order determines which group is designated with a suffix and which one with prefix and yes, it is true that if there is a functional group suffix and a substituent, the functional group suffix gets the lowest possible number.
The alcohol does have a higher priority and that is why the name has a suffix “ol” rather than “amine”. However, if we number in the opposite direction, the name would be 5-aminohexan-4-ol instead of 2-aminohexan-3-ol. In the first case, while the alcohol has a lower number than the amine, it is still on position 4 – it is a hexan-4-ol. In the second option, the alcohol has a higher number than the amine, which is not what we normally want, but nonetheless it still a hexan–3-ol. So, it is more of the alcohol competing with itself rather than the amine, and the option where it has a lower number is preferred despite the amine being numbered before it.
Another way to look at it is that it is not because of the amine that the numbering starts from where the NH2 is closer; it is because that is how the alcohol gets the lowest possible number.
If, for example, we separate them by one carbon, then the amino changes from 2-amino to 5-amino since that is how the OH keeps position 3 to be haxan-3-ol.
Honestly speaking this a very explained write up, and I said very great full for this note . Thank you so much and keep doing the good work.
I really learnt a lot from the content of the page. Thank you for the lesson.
this was very helpful thank you so much