Thiols are sulfur analogues of alcohols, containing an SH group in place of the OH group. When naming a thiol, we follow the same rules we discussed earlier for the IUPAC nomenclature rules for alcohols, with the focus on giving the carbon bonded to the SH group the lowest locant.
This would be a brief summary for naming a thiol:
Step 1. Identify the parent chain.
Step 2. Identify the substituents.
Step 3. Number the parent chain, giving the carbon bonded to the SH group the lowest locant
Step 4. Put everything together, having the substituents in alphabetical order.
The presence of the SH group is identified by changing the parent suffix from “e” to “thiol”:
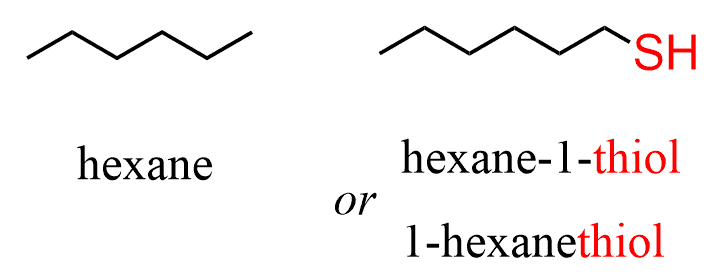
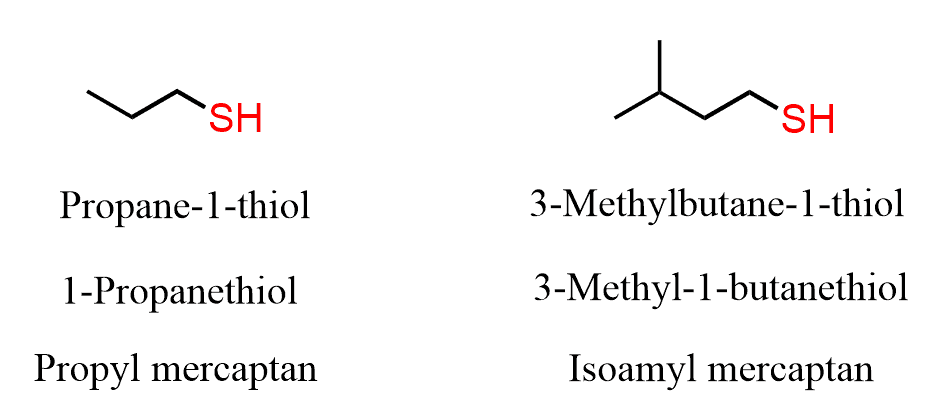
For simple thiols, the common name can be derived by naming the alkyl group connected to the SH and adding the word mercaptan:
If it is a branched molecule, choose the parent chain such that it is the longest carbon chain containing the carbon atom connected to the SH group:

The SH group has a higher priority than alkyl substituents or π bonds. Therefore, you need to number the parent chain such that the SH gets the lowest possible number:

If the SH group is connected to a chiral center, you will also need to include the absolute configuration at the beginning of the name. Also, if there is a double, the E and Z configuration should be addressed when applicable:

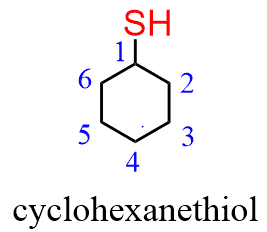
When naming a cyclic thiol, start numbering the ring beginning with the carbon connected to the SH group. This rule always puts the SH group at C1; therefore, the “1” is usually omitted from the name:
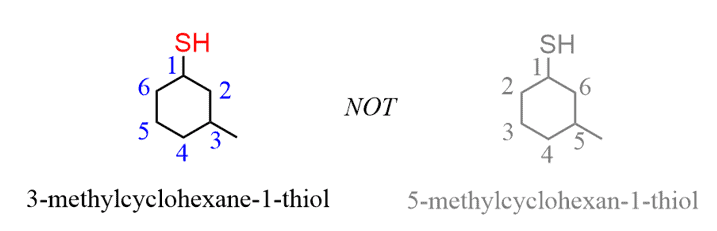
When other groups are present on the ring, it is numbered clockwise or counterclockwise depending on which direction gives the next substituent the lower number:
Thiols can also be identified by their common name, mercaptans, which literally means mercury capturing. This comes from the discovery of their strong affinity to heavy metal cations such as arsenic, which leads to forming strong complexes with them:

Compounds Where the Thiols are Not the Highest Priority
If the molecule contains a functional group with a higher priority than the thiol, the latter is mentioned as a substituent. The molecule in this case gets the suffix from the other functional group, while the prefix “mercapto” is used to mention the SH substituent.
For example, the hydroxyl takes precedence over the thiol, and therefore, we use the suffix “ol” and prefix mercapto:
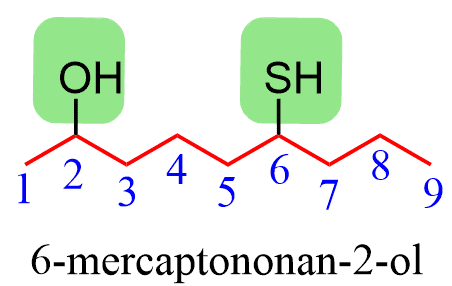
Alternatively, the SH group may be indicated by the prefix “sulfanyl”.
For determining the priorities of functional groups in a molecule, refer to the corresponding table, which can be found here.
Naming Sulfides
Sulfides are the sulfur analogs of ethers and are, therefore, also called thioethers.
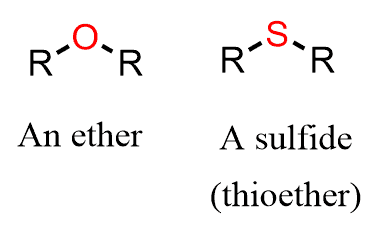
Just like ethers, sulfides are named by both common and systematic nomenclature of the IUPAC rules. The common names are used for sulfides with simple alkyl groups. To do this, we first identify the alkyl groups and arrange them in alphabetical order, followed by the word “sulfide”.
For example,
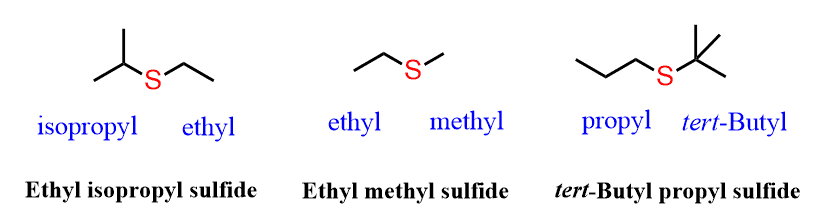
The molecules above are examples of unsymmetrical sulfides i.e., different alkyl groups are bridged with the sulfur.
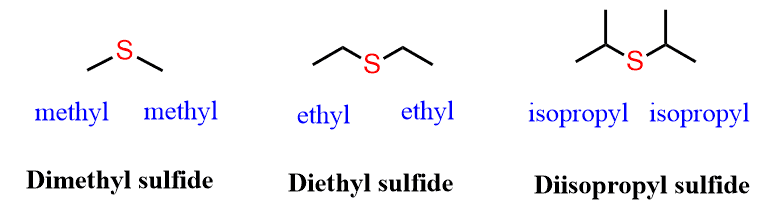
If the groups are identical – symmetrical sulfides, the prefix “di” is added. For example,
The Systematic Nomenclature of Sulfides
The systematic nomenclature is used for sulfides with complex substituents. The idea here is to treat one of the alkyl groups with the sulfur as a substituent connected to a parent chain. This substituent is named as “alkylsulfanyl” or “alkylthio”, shown in parentheses. The parent chain is determined just like we always do, based on the longest carbon chain.
For example,

Check Also
- Nomenclature of Alcohols: Naming Alcohols based on IUPAC Rules with Practice Problems
- Preparation of Alcohols via Substitution or Addition Reactions
- Reaction of Alcohols with HCl, HBr, and HI Acids
- Mesylates and Tosylates as Good Leaving Groups
- SOCl2 and PBr3 for Conversion of Alcohols to Alkyl Halides
- Alcohols in Substitution Reactions Practice Problems
- POCl3 for Dehydration of Alcohols
- Dehydration of Alcohols by E1 and E2 Elimination
- The Oxidation States of Organic Compounds
- LiAlH4 and NaBH4 Carbonyl Reduction Mechanism
- Alcohols from Carbonyl Reductions – Practice Problems
- Grignard Reaction in Preparing Alcohols with Practice Problems
- Grignard Reaction in Organic Synthesis with Practice Problems
- Protecting Groups For Alcohols in Organic Synthesis
- Oxidation of Alcohols: PCC, PDC, CrO3, DMP, Swern, and All of That
- Diols: Nomenclature, Preparation, and Reactions
- NaIO4 Oxidative Cleavage of Diols
- The Pinacol Rearrangement
- The Williamson Ether Synthesis
- Alcohol Reactions Practice Problems
- Reactions of Thiols
- Alcohols Quiz – Naming, Preparation, and Reactions
- Reactions Map of Alcohols
