In this conversion series, we mention how nitriles can be converted to ketones and aldehydes. Nitriles are converted to ketones by both Grignard and organolithium reagents:

The new C-C bonds are formed by nucleophilic addition of the organometallic reagents to the polar C-N triple bond. After protonation of the resulting anion, an imine is formed, which is then hydrolyzed to a ketone.
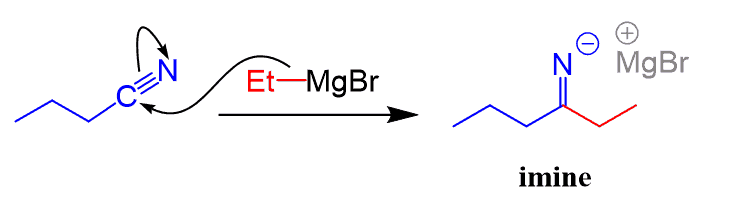
Notice that this is different from the Grignard reaction of aldehydes, ketones, esters, and acid chlorides where the major product was an alcohol.
The reason for this difference is that the negatively charged imine cannot go another nucleophilic addition since that would place two negative charges on the nitrogen.
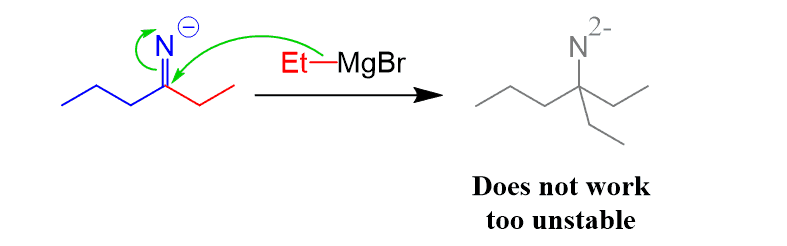
The mechanisms of the nitrile reaction with Grignard and organolithium are covered in a separate post.
Nitriles to Aldehydes
Nitriles can be converted to aldehydes by a reduction with DIBAL or hydrolysis using SnCl2 catalyst with HCl (Stephen aldehyde synthesis).
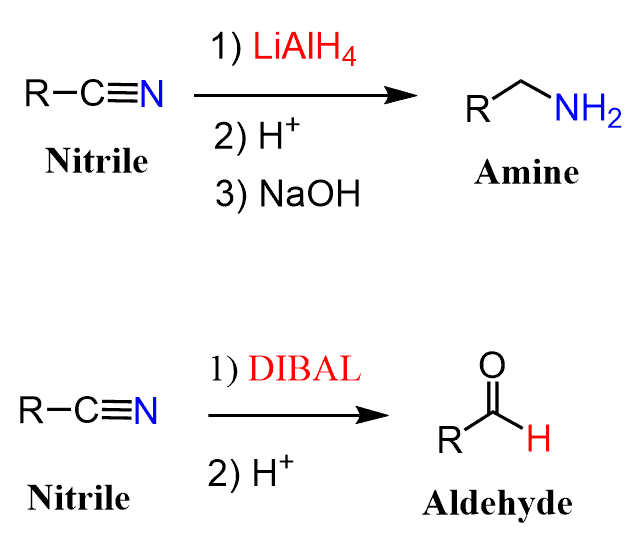
Notice that the reaction of nitriles with a stronger reducing agent LiAlH4, yields primary amines.
Both of these mechanisms are covered in detail here.
Organic Chemistry Reaction Maps
Never struggle again to figure out how to convert an alkyl halide to an alcohol, an alkene to an alkyne, a nitrile to a ketone, a ketone to an aldehyde, and more! The comprehensive powerfull Reaction Maps of organic functional group transformations are here!
Check Also
- Preparation of Carboxylic Acids
- Naming Carboxylic Acids
- Naming Nitriles
- Naming Esters
- Naming Carboxylic Acid Derivatives – Practice Problems
- Fischer Esterification
- Ester Hydrolysis by Acid and Base-Catalyzed Hydrolysis
- What is Transesterification?
- Esters Reaction with Amines – The Aminolysis Mechanism
- Ester Reactions Summary and Practice Problems
- Preparation of Acyl (Acid) Chlorides (ROCl)
- Reactions of Acid Chlorides (ROCl) with Nucleophiles
- Reaction of Acyl Chlorides with Grignard and Gilman (Organocuprate) Reagents
- Reduction of Acyl Chlorides by LiAlH4, NaBH4, and LiAl(OtBu)3H
- Preparation and Reaction Mechanism of Carboxylic Anhydrides
- Amides – Structure and Reactivity
- Naming Amides
- Amides Hydrolysis: Acid and Base-Catalyzed Mechanism
- Amide Dehydration Mechanism by SOCl2, POCl3, and P2O5
- Amide Reduction Mechanism by LiAlH4
- Amides Preparation and Reactions Summary
- Amides from Carboxylic Acids-DCC and EDC Coupling
- The Mechanism of Nitrile Hydrolysis To Carboxylic Acid
- Nitrile Reduction Mechanism with LiAlH4 and DIBAL to Amine or Aldehyde
- The Mechanism of Grignard and Organolithium Reactions with Nitriles
- Carboxylic Acids and Their Derivatives Practice Problems
- Carboxylic Acids and Their Derivatives Quiz

