In the previous two posts, we have talked about the number of NMR signals and the multiplicity which is the signal splitting based on the n + 1 rule.
Let’s quickly summarize what these two features are.
First, the number of signals:
In short, the number of signals is equal to the number of protons in different environments. These are the nonequivalent protons.
The easiest/fastest method is the check of symmetry elements according to this flowchart:
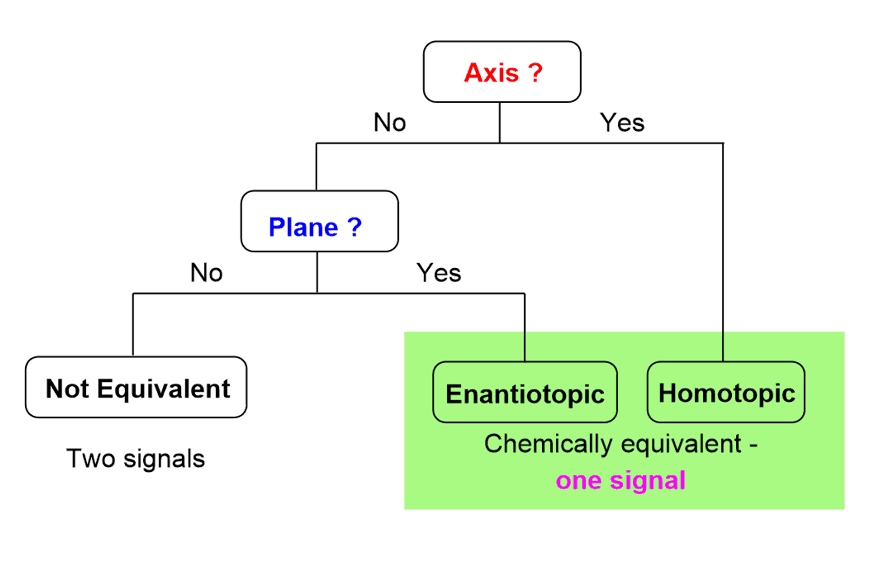
Second, the signal splitting:
The number of peaks in a given NMR signal depends on the number of adjacent protons and is calculated based on the n + 1 rule.
Here is a summary table for the splitting patterns in NMR spectroscopy.

Read the above-mentioned articles for more details if you need to and if you are ready, scroll down and work on these problems.




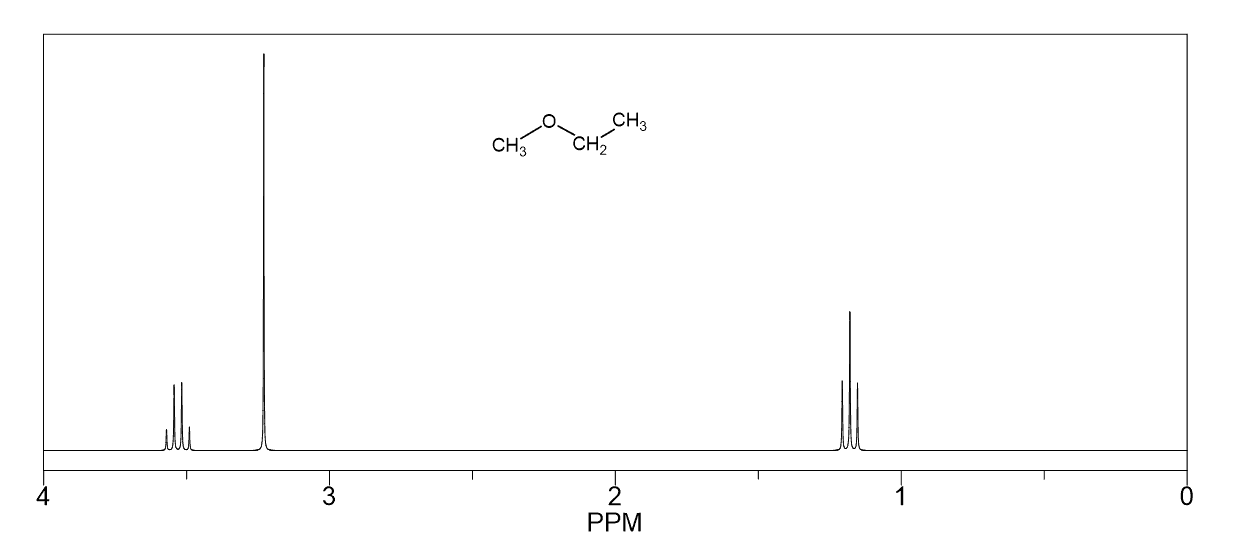
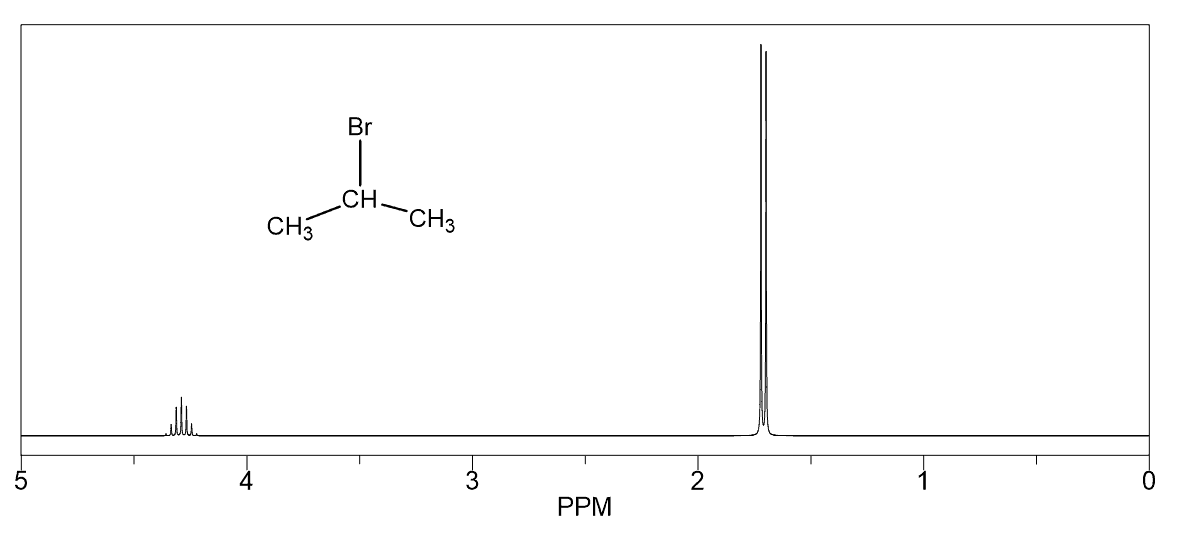
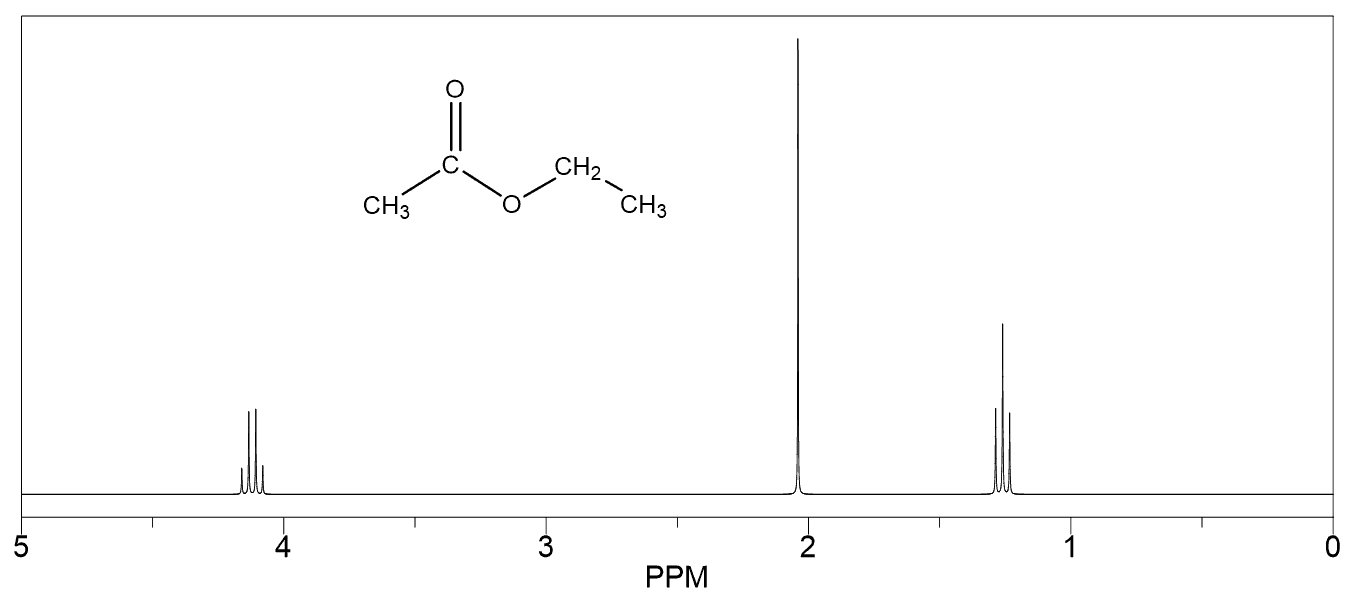
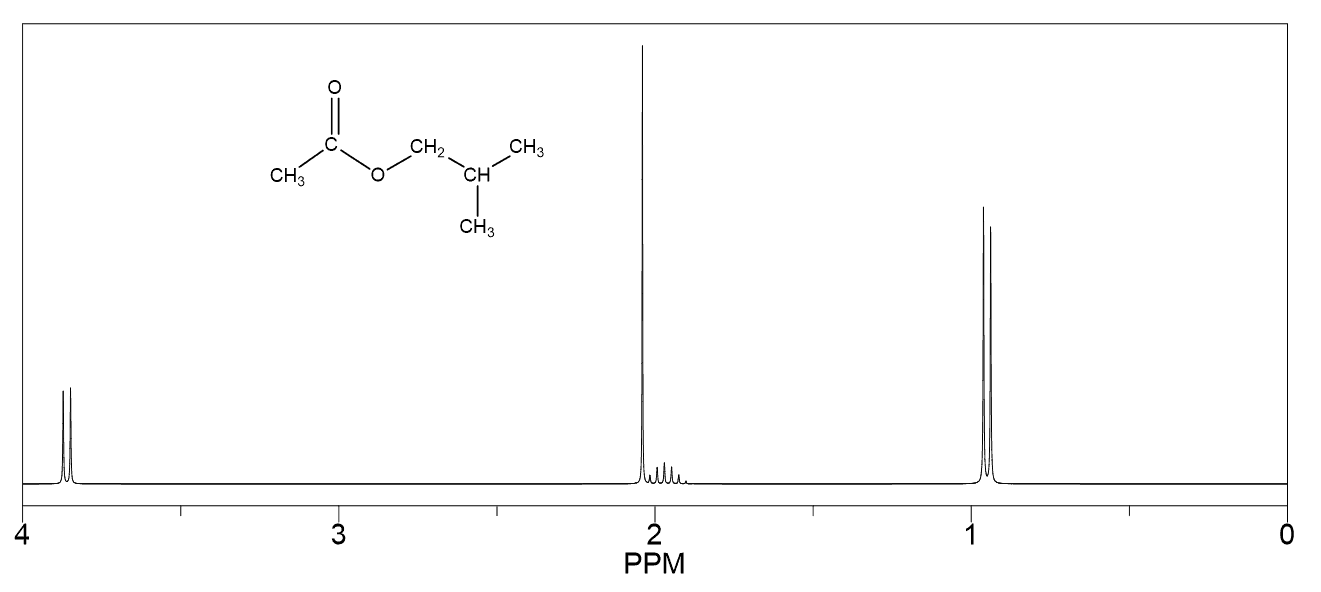
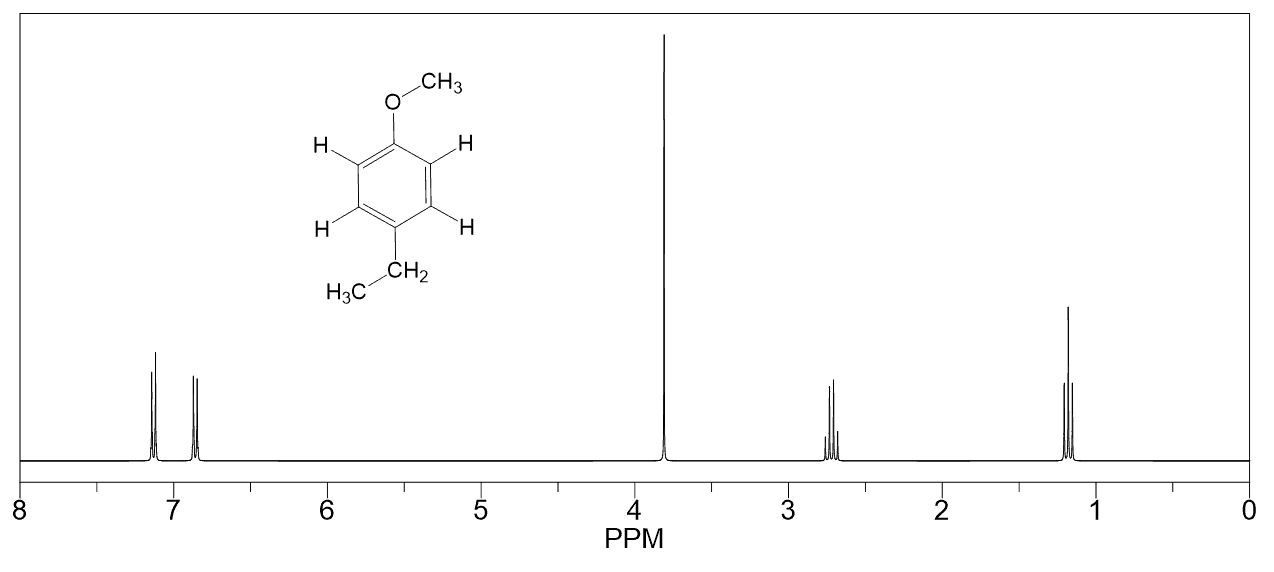
Hello!
For #7, wouldn’t protons b more downfield than protons c since they are closer to the oxygen (an electron withdrawing group)?
thanks 🙂
Hello Olivia,
That’s a good argument for predicting which of the protons next to the oxygen will be more downfield. Yes, oxygen is more electronegative than carbon and for that reason, one may expect to have the protons on the adjacent carbon (ortho position if you have covered aromatic chemistry) to be more deshielded and thus more downfield.
However, there is also the resonance effect of the oxygen since its lone pairs are donating electron density through conjugation with the aromatic pi system. This is the reason why the ortho and para position of resonance-activated benzene rings are more electron-rich and therefore, more reactive in electrophilic aromatic substitution. Drawing resonance structures should help to visualize this better:
To summarize this, when an electron-donating group is present, the ortho and para protons are shifted upfield because of the increased electron-density in these positions.
In contrast, if instead of the methoxy group, there was another one that could only affect the electron density of the ring through the inductive effect, then yes, the protons on the otho position (protons “b” in this problem would have been more deshielded and resonate at higher frequency-downfield.
Let me know if you have other questions!
Hi I was wondering how do you know how to predict a chemical shift value when it is next to an atom that will de-sheild the proton. For example, if a methyl group was next to a Br atom, that will probably change the chemical shift value a little higher ? I hope my question makes sense.
Hello,
The chemical shift changes are based on the electron density around the proton. In general, the more electronegative the neighboring atom(s) the higher the ppm value (downfield). This is due to the higher electronegativity of those atoms pulling the electron density and deshielding the protons. As a result, they are more exposed to the magnetic field and require higher energy radiation for resonance absorption.
As to predicting the value, there is no need to know the exact values, and I don’t think you will be asked to do so. However, the general trends and important areas of NMR spectra are certainly needed to be recognized.
Have you checked the following two artciles?
NMR Chemical Shift – ppm, Upfield, Downfield
NMR Chemical Shift Values Table