Aldoses contain alcohol and aldehyde functional groups, which can be oxidized to carboxylic acids. Depending on the oxidizing agent, we can either selectively oxidize the aldehyde or involve the primary alcohol as well. If both groups are oxidized to a carboxylic acid, an aldaric acid is formed, while the selective oxidation of the aldehyde results in an aldonic acid:

Using a mild oxidizing agent allows distinguishing aldoses and ketoses since the aldehyde, being more reactive, is converted to a carboxylic acid, while the ketone and the alcohols present in both are not. Br2 is an ideal candidate for this, as its red color disappears upon reduction to Br− when reacted with the aldehyde. Although the mechanisms are different, the color loss behind the test is much like what we learn in the bromination reaction of alkenes.

If the monosaccharide is, on the other hand, a ketose, the red color will persist, indicating no reaction with Br2.
One thing to keep in mind, though, is the isomerization between aldoses and ketoses that occurs in basic conditions. If the ketose is isomerized to an aldose, it will give a positive signal in the Br2 test. To prevent this, the reaction with Br2 is usually carried out at slightly acidic conditions (pH = 6).
There are different ways of oxidizing both aldoses and ketoses performed under basic conditions to promote the isomerization:
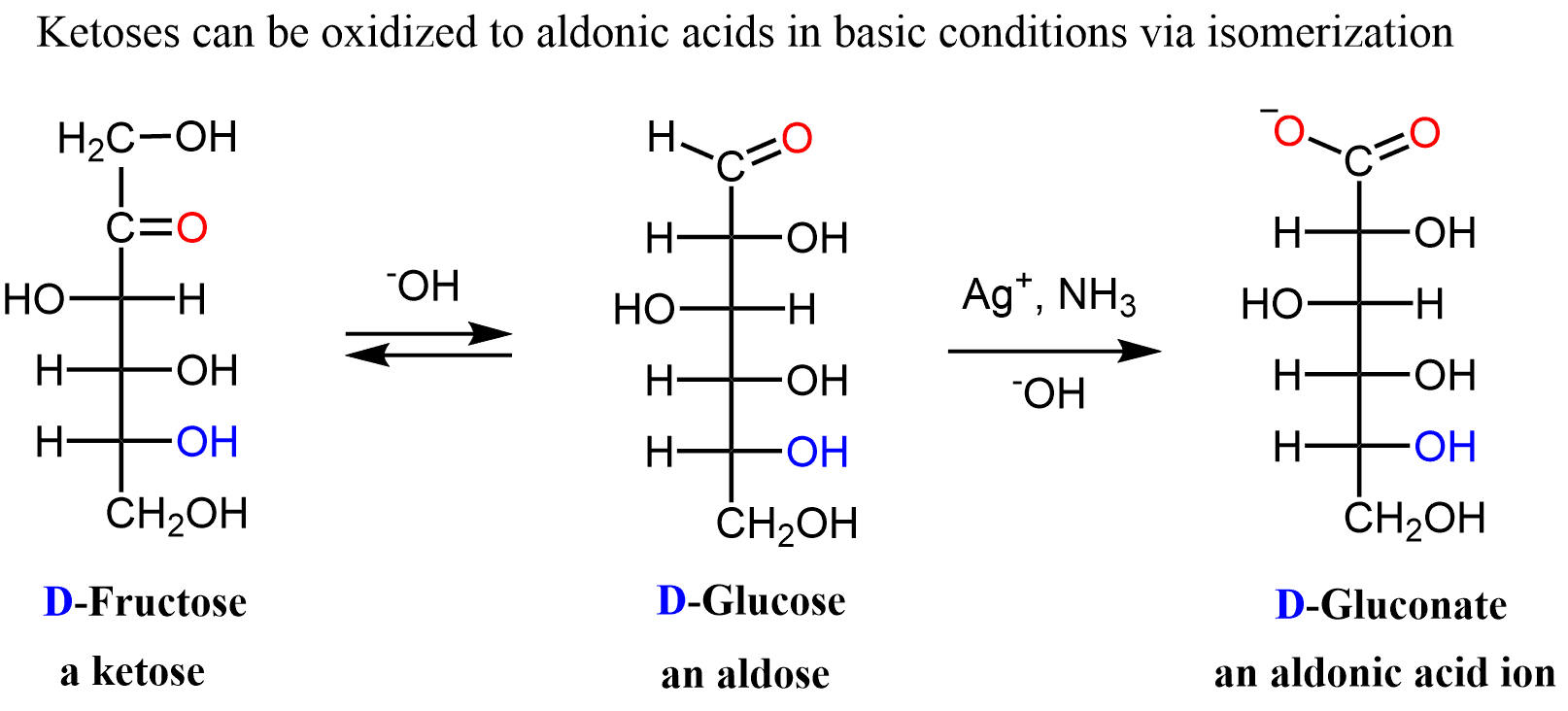
The most common examples are the Tollens’ reagent (Ag+ NH3, HO−), Fehling’s reagent (Cu2+ in aqueous sodium tartrate), and Benedict’s reagent (Cu2+ in aqueous sodium citrate).

The indicator in Tollens reagent is the by-product Ag, which coats the surface of the flask, forming a mirror:

The Fehling’s and Benedict’s tests rely on the formation of Cu2O as a red precipitate when the Cu2+ salt is reduced by the aldose or ketose present in the solution:

A common way of oxidizing both the aldehyde and 1o alcohol groups to carboxylic acids (aldaric acid) is the use of nitric acid (HNO3), which is a stronger oxidizing agent. This reaction, however, is not a test but rather a method for converting the functional groups:

One feature of aldaric acids is that they have two identical groups (COOH) on the terminal carbons, and therefore, it is always a good idea to check whether it is a meso compound. For example, D-Glucose is chiral while D-glucaric acid is a meso compound, as can be seen from the perpendicular plane of symmetry on the Fischer projection. Remember, meso compounds are not optically active and therefore are achiral.

Reducing and Nonreducing Sugars
Recall from general chemistry that any redox reaction involves an oxidation and reduction, so if one species is oxidized, then the other is reduced.
Now, in all the reactions above, the carbohydrates were oxidized and therefore, the oxidizing agents were reduced. So, the oxidizing agents are reduced by the carbohydrates, and because of this, any carbohydrate that gives a positive test with Tollens, Benedict’s, or Fehling’s reagent is called a reducing sugar. Consequently, carbohydrates that do not react with these oxidizing reagents are classified as nonreducing sugars.
You may wonder what some examples of non-reducing sugars are, then. To have a general answer to this question, first, remember that cyclic forms of sugars do not have the aldehyde functional group, but rather they are in the hemiacetal or acetal form in the case of glycosides.
Remember also that cyclic hemiacetals are always in equilibrium with the open-chain form and therefore, they can also be oxidized to aldonic or aldaric acids – they are reducing sugars:

Glycosides, on the other hand, are nonreducing sugars since they do not undergo ring-opening reactions under neutral or basic conditions.

Need some practice on carbohydrates?
Check this Multiple-Choice, summary quiz on the structure and reactions of carbohydrates with a 40-minute video solution!
Carbohydrates Practice Problem Quiz
Check also in Carbohydrates
- Carbohydrates – Structure and Classification
- Erythro and Threo
- D and L Sugars
- Aldoses and Ketoses: Classification and Stereochemistry
- Epimers and Anomers
- Converting Fischer, Haworth, and Chair forms of Carbohydrates
- Mutarotation
- Glycosides
- Isomerization of Carbohydrates
- Ether and Ester Derivatives of Carbohydrates
- Oxidation of Monosaccharides
- Reduction of Monosaccharides
- Kiliani–Fischer Synthesis
- Wohl Degradation

