In oxidative cleavage reactions, C=C bonds are broken and replaced with C=O bonds. In this article, we will specifically focus on the oxidative cleavage of alkenes. This is most commonly achieved with potassium permanganate (KMnO4) and ozonolysis:

So, we have two processes here: the first is the oxidation of the carbon atoms since they make bonds with oxygen, and the second is the breaking of C-C bonds, which is what we refer to as “cleavage”.
As a side note, remember that treating alkenes with a cold solution of KMnO4 leads to syn dihydroxylation:

Oxidative Cleavage by Ozonolysis
The ozonolysis, on the other hand, can be carried out with an oxidative or reductive work-up. And, most often, what we learn in the ozonolysis of alkenes is the reductive workup of the ozonide cyclic intermediate by dimethyl sulfide (Me2S). This results in the formation of an aldehyde or a ketone, depending on the structure of the alkene:

Now, how does using a peroxide instead of DMS (Me₂S) change the product from an aldehyde to a carboxylic acid? The key difference is that the peroxide oxidizes the aldehyde into the corresponding carboxylic acid. As in regular ozonolysis, an unstable ozonide intermediate is formed first, which decomposes to give the aldehyde. The subsequent oxidation of this aldehyde to a carboxylic acid is shown in the mechanism below.
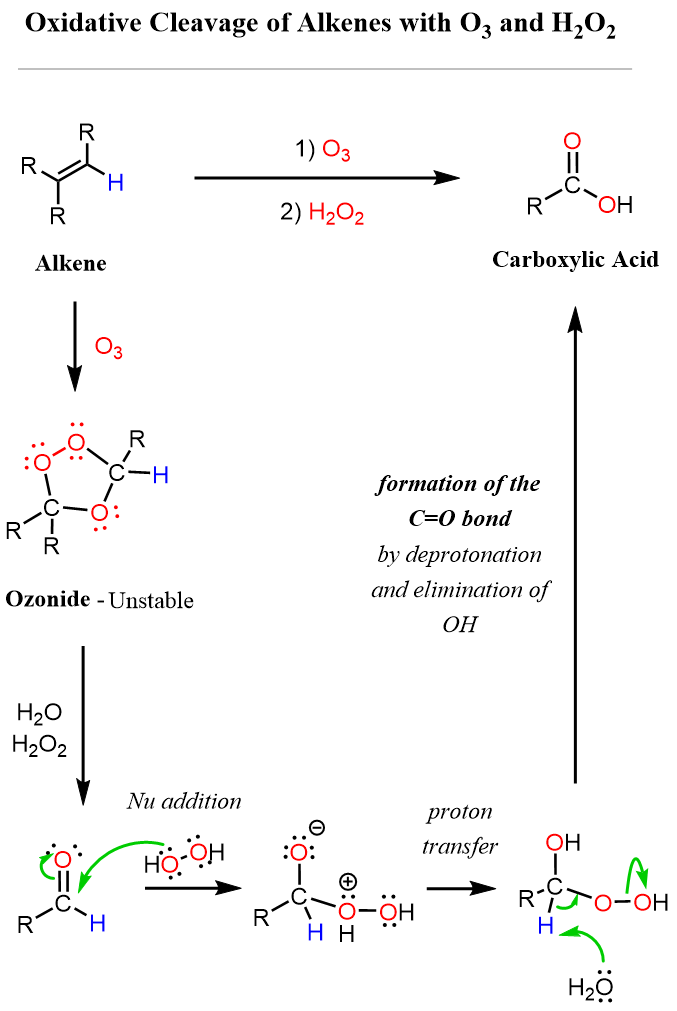
As shown in the first set of examples, the other half of the alkene that carries two alkyl groups (R) is converted directly into a ketone upon decomposition of the ozonide intermediate. Recall that ketones cannot be further oxidized because they lack the required hydrogen attached to the carbonyl carbon. For more details, see this article on the oxidation mechanisms of various carbonyl groups and alcohols.
Oxidative Cleavage by KMnO4
The oxidative cleavage by KMnO4 starts with an addition to the π bond, forming a cyclic intermediate which eventually breaks down to an aldehyde or a ketone.

The aldehyde is further oxidized to a carboxylic acid by KMnO4. The mechanism of this transformation is covered in the oxidation of alcohols.
Check Also
- Electrophilic Addition Reactions to Alkenes
- Markovnikov’s Rule
- Markovnikov’s Rule with Practice Problems
- Addition of Water to Alkenes
- Acid-Catalyzed Hydration of Alkenes with Practice Problems
- Rearrangements in Alkene Addition Reactions
- Oxymercuration-Demercuration
- Addition of Alcohols to Alkenes
- Free-Radical Addition of HBr: Anti-Markovnikov Addition
- Hydroboration-Oxidation: The Mechanism
- Hydroboration-Oxidation of Alkenes: Regiochemistry and Stereochemistry with Practice Problems
- Halogenation of Alkenes and Halohydrin Formation
- The Regiochemistry of Alkene Addition Reactions
- The Stereochemistry of Alkene Addition Reactions
- Cis product in an anti-Addition Reaction of Alkenes
- Ozonolysis of Alkenes with Practice Problems
- Syn Dihydroxylation of Alkenes with KMnO4 and OsO4
- Anti-Dihydroxylation of Alkenes with MCPBA and Other Peroxides with Practice Problems
- Alkene Reactions Practice Problems
- Changing the Position of a Double Bond
- Changing the Position of a Leaving Group
- Alkenes Multi-Step Synthesis Practice Problems
- Alkene Addition Reactions Practice Quiz
- Reactions Map of Alkenes

your blog is really informative and effective.it’s very clear and easy to understand. Good going keep it up.
This is great, thanks.