Depending on whether you need to oxidize a primary alcohol to an aldehyde or a carboxylic acid, you can use either pyridinium chlorochromate (PCC) or Jones reagent (a solution of chromium trioxide, CrO3 in aqueous sulfuric acid):
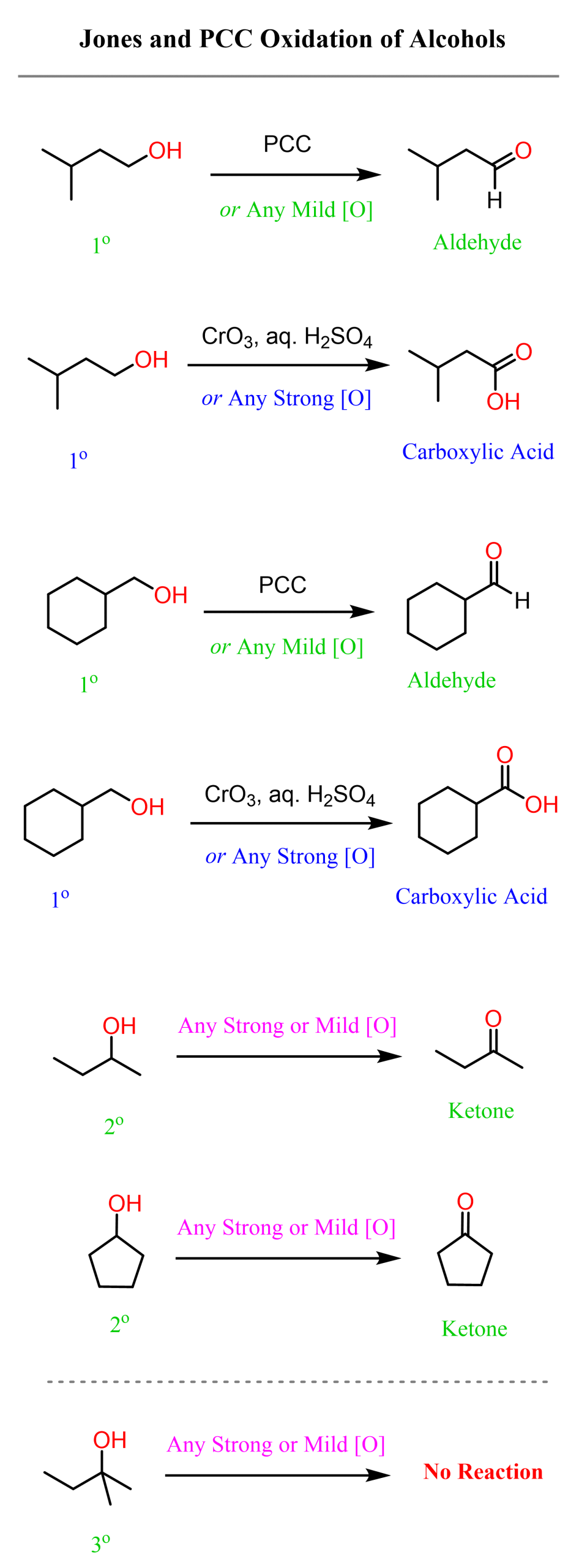
Notice that the oxidation to carboxylic acids only happens when a primary alcohol is used. Secondary alcohols can only be oxidized to the corresponding ketone regardless of whether a strong or mild oxidizing agent is used.
Recall that carboxylic acids are higher up in the scale of oxidation states in organic chemistry; therefore, we can say that PCC is a mild oxidizing agent, while Jones is strong, as it oxidizes primary alcohols all the way to carboxylic acids:

Aside from PCC, there are a few other mild oxidizing agents such as pyridinium dichromate (PDC), Swern oxidation using DMSO, (COCl)₂ and Et₃N, and the Dess–Martin (DMP). Here is the list of the most common strong and mild oxidizing agents you will see in your organic chemistry class:

Check out this post for a comprehensive coverage of all the oxidizing agents with the corresponding mechanisms. The mechanism of Jones oxidation with CrO3 and H2CrO4 is also covered in this post.


