When 1,2-diols (vicinal diols) are treated with acid, a rearrangement takes place, and the diol is transformed to a ketone.

This reaction is known as the pinacol rearrangement since pinacol is the simplest diol capable of undergoing such a transformation.
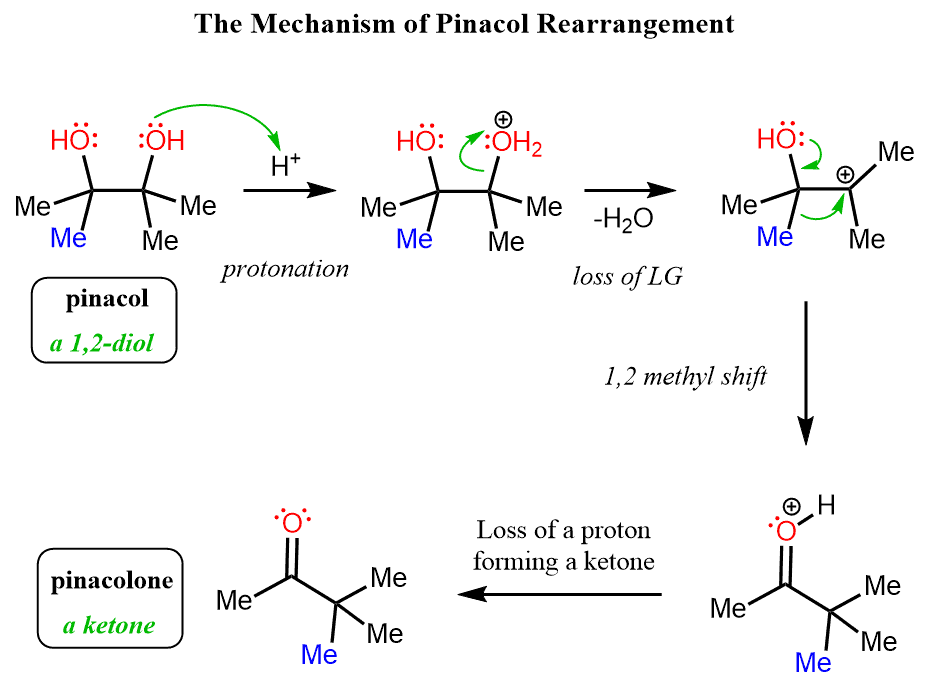
Let’s understand how this rearrangement occurs. From the nucleophilic substitution and elimination reactions, we know a rearrangement presumes the formation of a carbocation. So, how can a carbocation be generated from pinacol? The only option here is to protonate one of the OH groups and expel it as water, leaving behind a positively charged carbon atom.
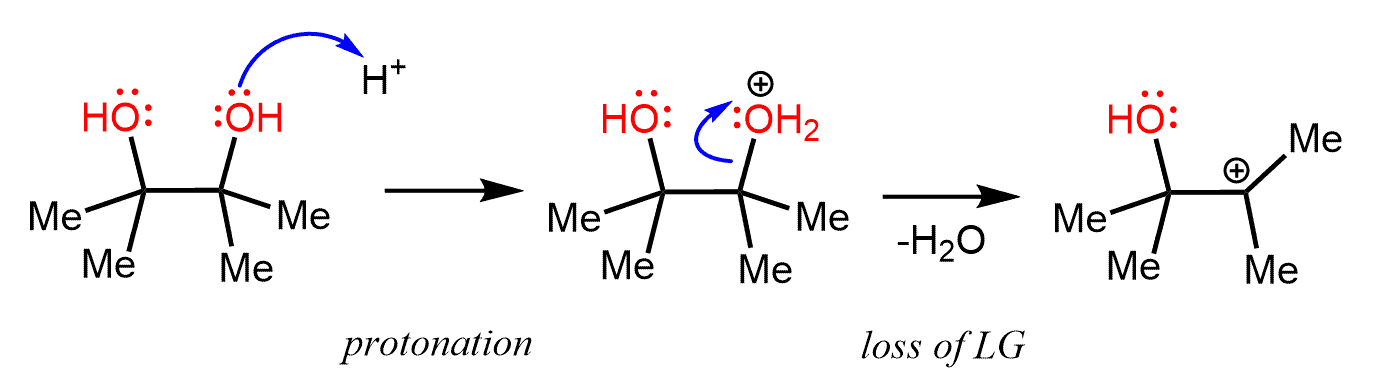
Now, the next question is, why does this rearrangement take place? In general, a rearrangement happens only if the resulting carbocation is more stable than the original one. Remember that more substituted carbocations are more stable because of the electron-donating effect of alkyl groups and the hyperconjugation.

In this case, the carbocation that is formed after the loss of water is already a tertiary carbocation, and the question is, what is more stable than the tertiary carbocation that will be the driving force for this rearrangement?
So, what other factors do you know about stabilizing a positive charge?
Can you think of a way to involve oxygen in this process?
Remember, in the article about resonance stabilization, we mentioned that oxygen is very good at stabilizing a positive charge on an adjacent atom. This happens through the delocalization of the lone pairs on the oxygen:
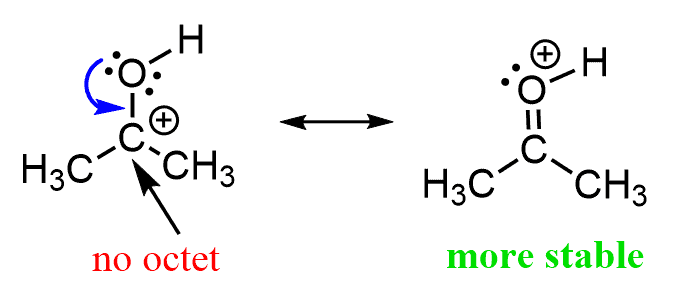
Even though oxygen is more electronegative, and it is unfavored to put a positive charge on it, the resonance structure on the right is more stable since all the atoms there have a complete octet.
Going back to our carbocation, we can see that as it stands, there is no possibility to involve the oxygen in resonance-stabilization.
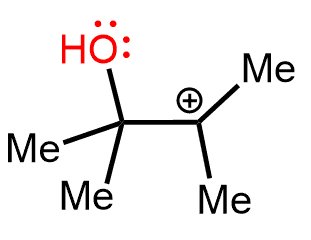
However, if a methyl shift takes place, the positive charge is now next to the oxygen and it is resonance-stabilized by its lone pairs:
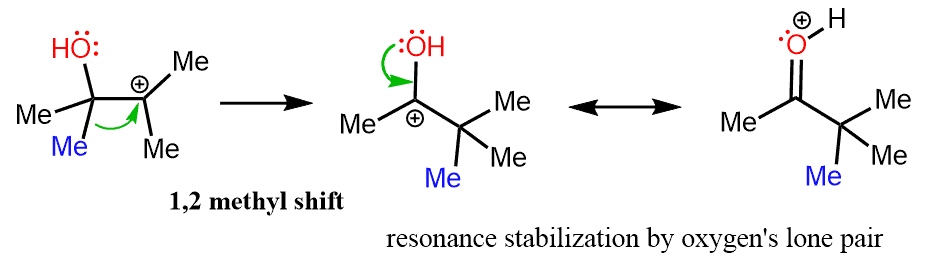
It might be more accurate to show the 1,2-methyl shift initiated by the lone pair on the oxygen:
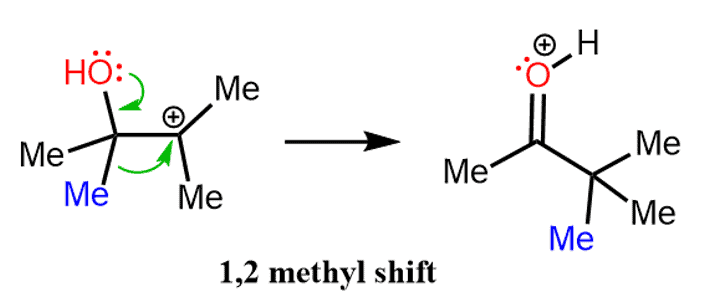
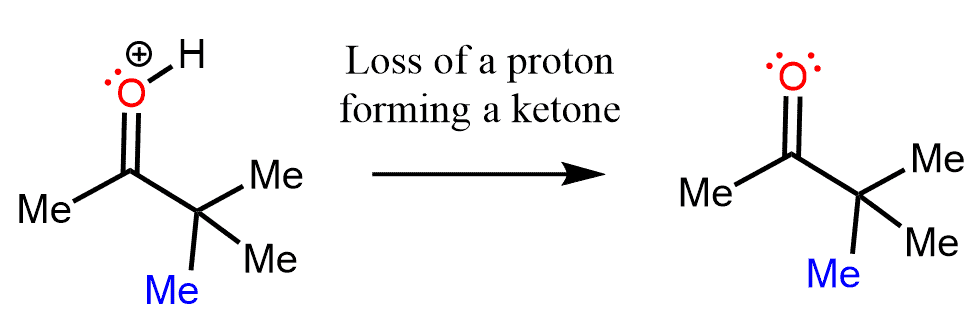
In the last step, a stable ketone is formed by the loss of a proton from the positively charged oxygen.
In summary, the mechanism of pinacol rearrangement can be shown as follows:
Pinacol Rearrangements of Unsymmetrical Diols
When we are working with a symmetrical diol, it doesn’t matter which of the oxygen atoms is protonated, and subsequently, the alkyl group that migrates.
However, if the diol is unsymmetrical, the reaction goes such that the more stable carbocation is formed.
For example, the following diol can undergo a pinacol rearrangement, and two possible ketones can be expected to form:
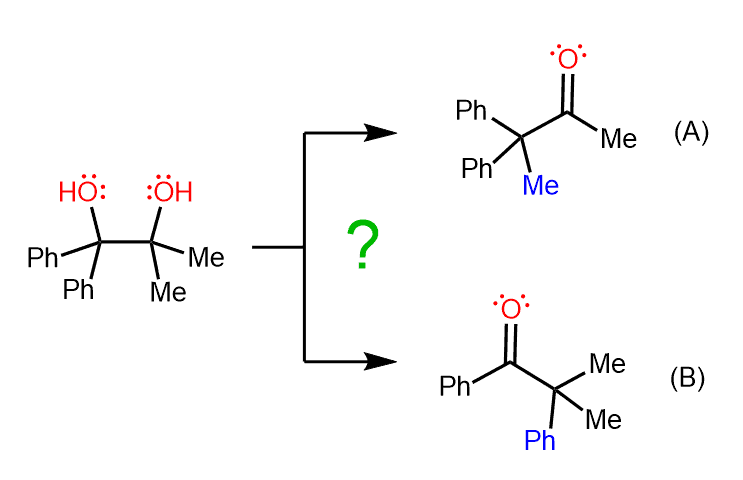
However, experiments have shown that only one of the ketones is formed, and that is compound (A).
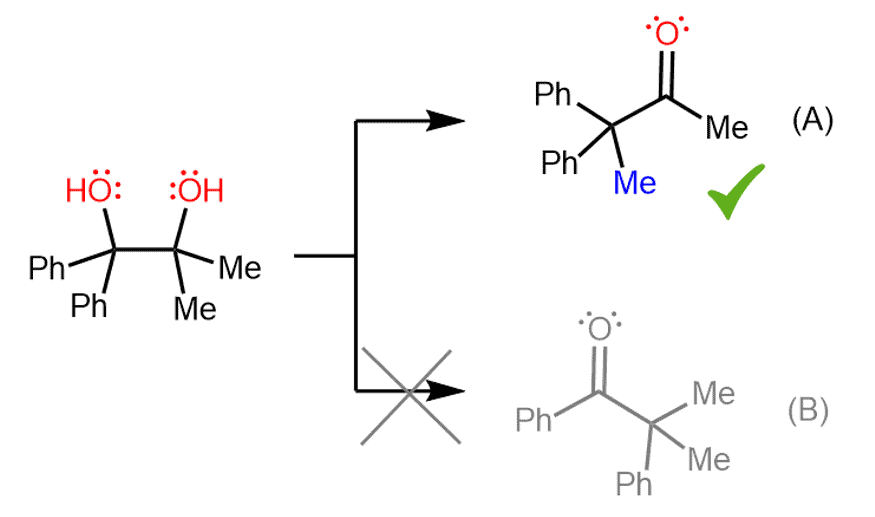
To explain this selectivity, we need to consider the carbocation that is formed after the loss of each OH group.

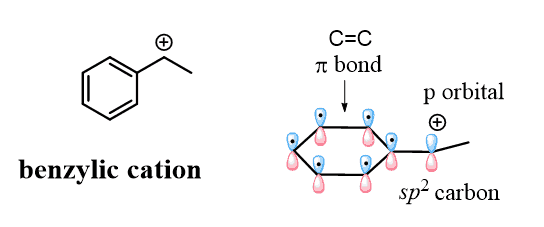
Here again, even though both carbocations are tertiary, the intermediate (1) is more stable because it is a benzylic carbocation, and we know that these are resonance-stabilized:

Remember that carbocations are sp2-hybridized and the empty p orbital of the positively charged carbon is nicely aligned with the p orbitals of the aromatic system, which makes the cation resonance-stabilized:
The preference for the more stable carbocation determines the major product of this pinacol rearrangement. Since carbocation (2) is not formed in a significant amount, the phenyl ketone originating from it is also not observed in the reaction products.
Instead, the carbocation stabilized by two adjacent phenyl groups is formed, and the consequent methyl shift gives ketone (A) as the major organic product.
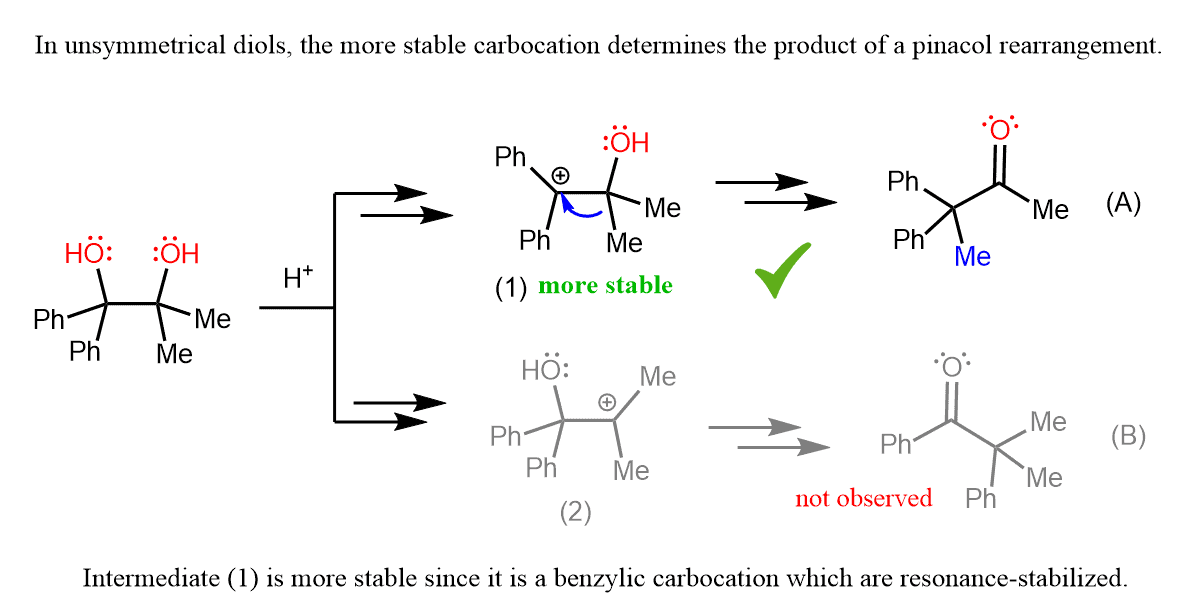
So, to summarize, when working with an unsymmetrical diol, remember that the product is to be determined based on the formation of the more stable carbocation.
Migratory Ability in the Pinacol Rearrangement
Let’s also consider a scenario of a pinacol rearrangement when there are two groups that can undergo a 1,2-shift to an electron-deficient center. For example, in the following diol, we will have competition of migratory aptitude (ability) of methyl and phenyl groups.
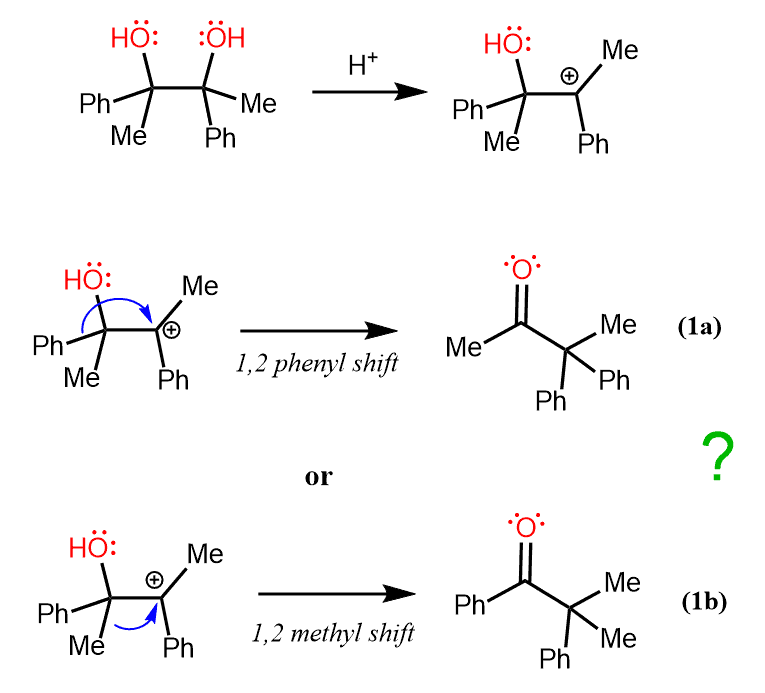
And it turns out that the phenyl group makes an easier 1,2 shift to the carbocation since ketone (1a) is the major product of this reaction:
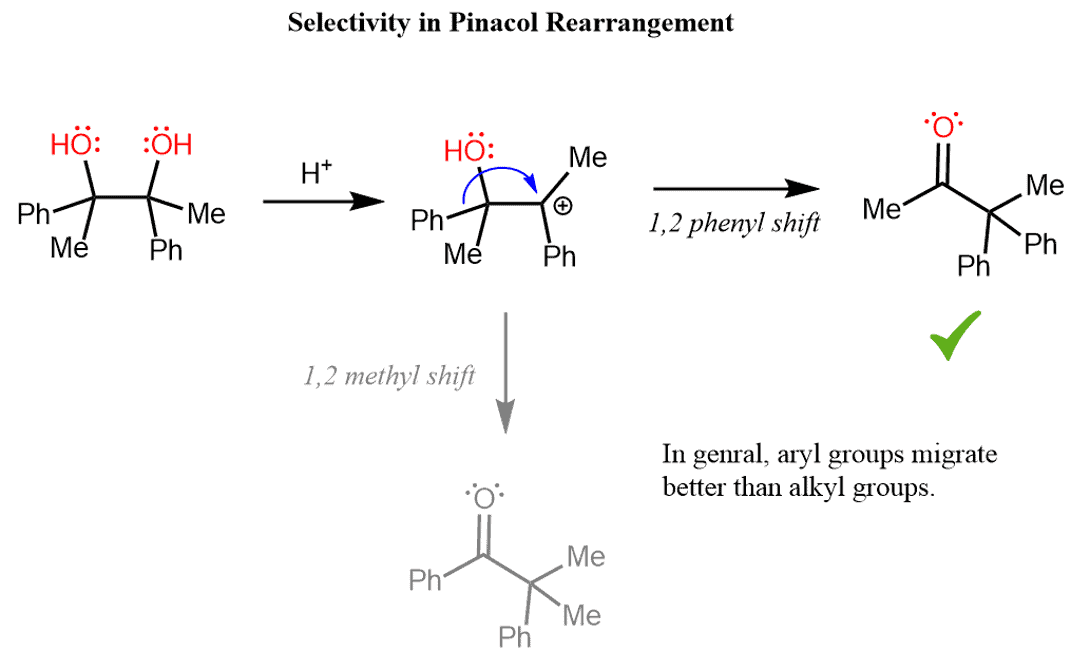
In general, it is not so straightforward to assess the relative migratory aptitude of since it does not solely depend upon the migratory ability of the given group. The overall process will also depend on which group(s) are left behind to stabilize the resulting carbocation. For example, in one reaction, methyl might migrate faster than ethyl since the resulting cation is resonance stabilized, which does not necessarily imply that methyl groups are always better at 1, 2 shifts.
Nevertheless, there are some general trends that can be concluded from experimental data. For a reference guide in assessing the migratory ability, remember that aryl groups > alkyl groups. As far as the hydrogen shifts, they have shown mixed results relative to aryl and alkyl groups. These mainly depend on the structure of the reactant and the experimental conditions.
Below are some practice problems on the pinacol rearrangement.


