Preparation of Anhydrides
Anhydrides are prepared by dehydration of carboxylic acids either at extremely high temperatures (800 oC) or by using P2O5 as a dehydrating agent:
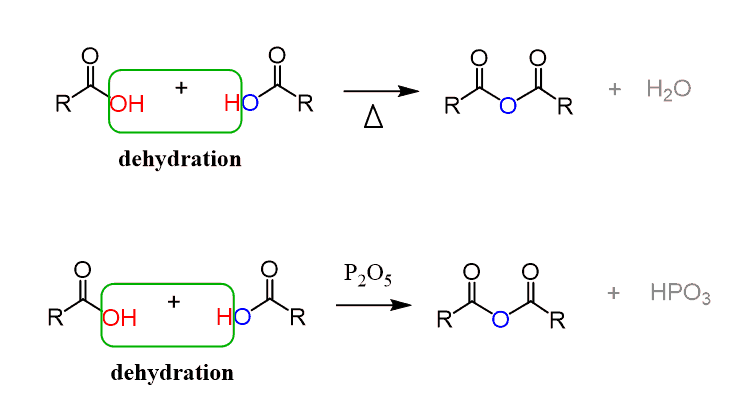
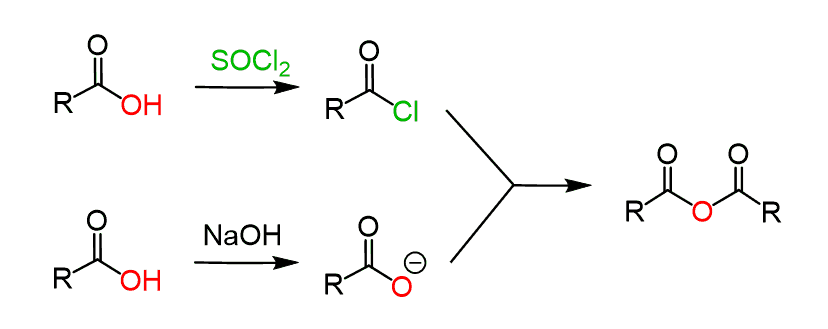
For most carboxylic acids are not compatible with excessive heating, and even using P2O5 is not a practically applicable solution. Therefore, the most common method for preparing carboxylic anhydrides in the laboratory is the conversion of the acid to an acid chloride and a carboxylate salt, which react readily at room temperature:
Reactions of Anhydrides
Although slightly less reactive than acid chlorides, anhydrides react with all the nucleophiles in an identical mechanism.
The only difference in these nucleophilic acyl substitutions is the change of the leaving group from chloride to a carboxylate.
Hydrolysis of Anhydrides
Anhydrides can be hydrolyzed to carboxylic acids. Adding a base would increase the rate of the conversion:

Esters from Anhydrides
Anhydrides can be converted into esters by using an alcohol with a base or, even better, an alkoxide which is a stronger nucleophile:

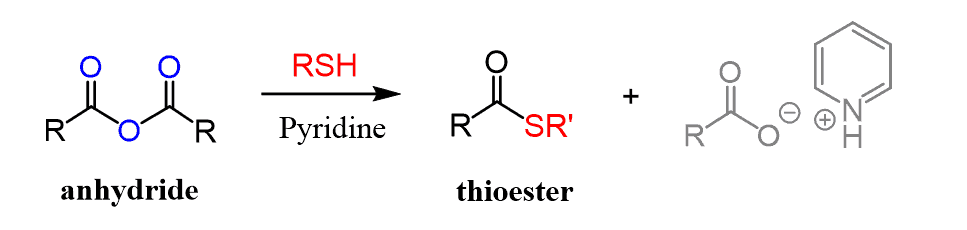
In a similar reaction, thioesters can be prepared from anhydrides:
Amides from Anhydrides
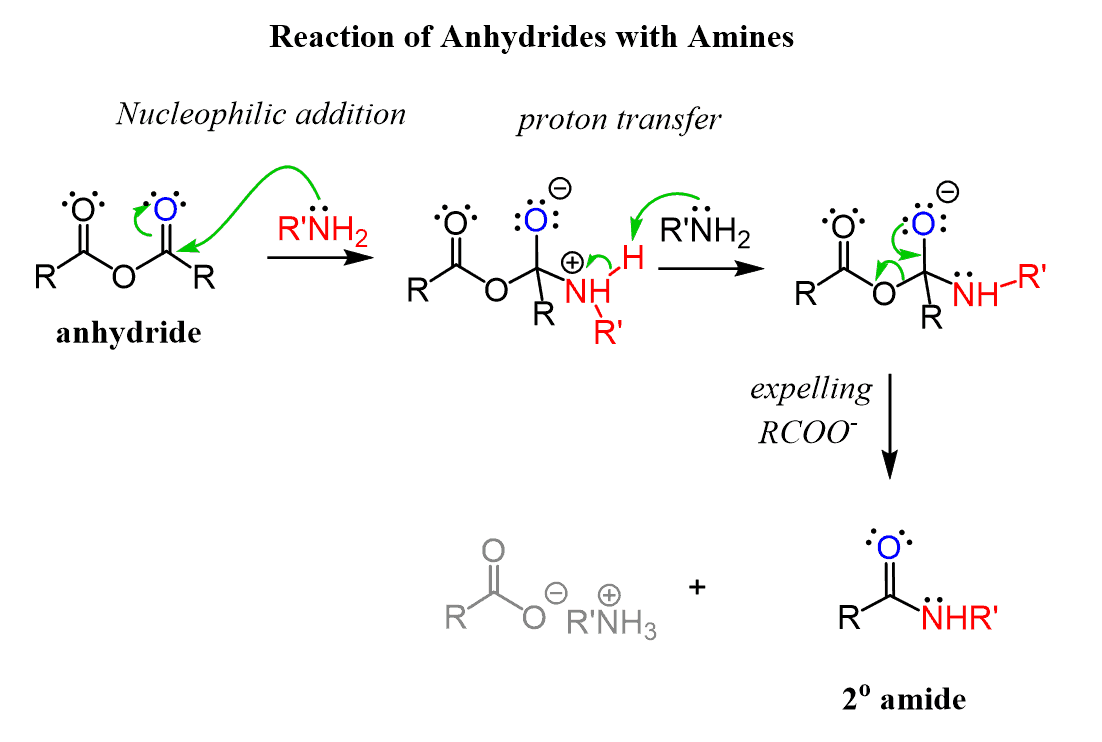
Amines, being good nucleophiles, react readily with anhydrides to form primary, secondary or tertiary amides:
Anhydrides in Grignard Reaction
Just like the acid chlorides and esters, anhydrides react with excess Grignard reagent, forming a tertiary alcohol:
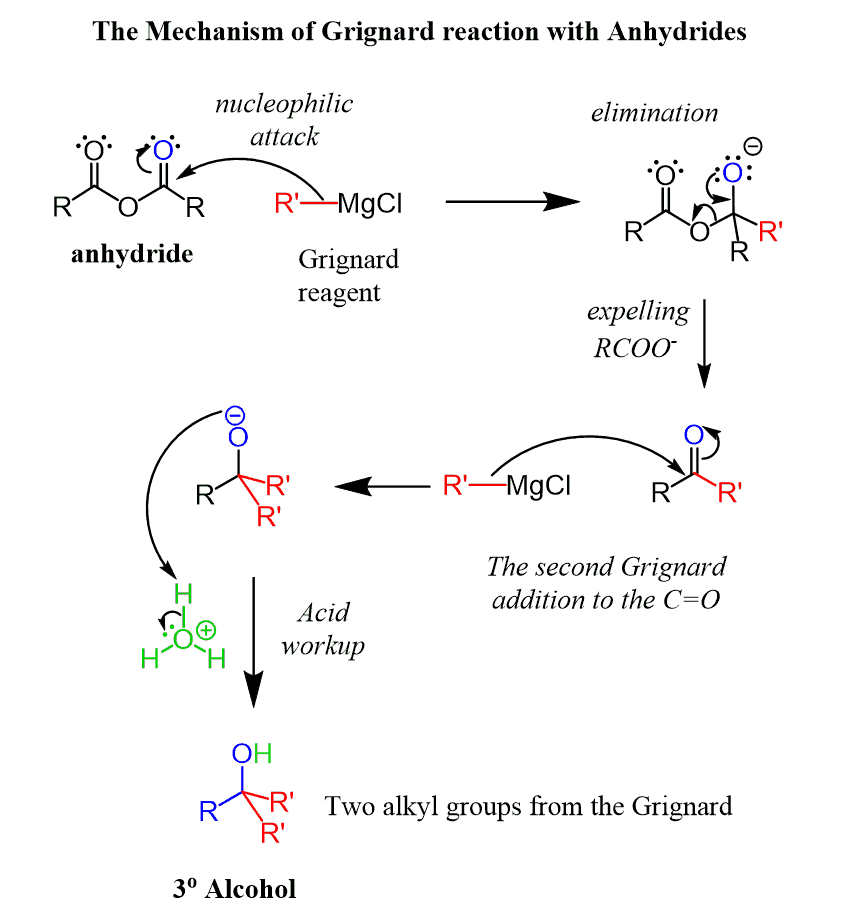
Reduction of Anhydrides
Anhydrides can be reduced to a primary alcohol using LiAlH4:
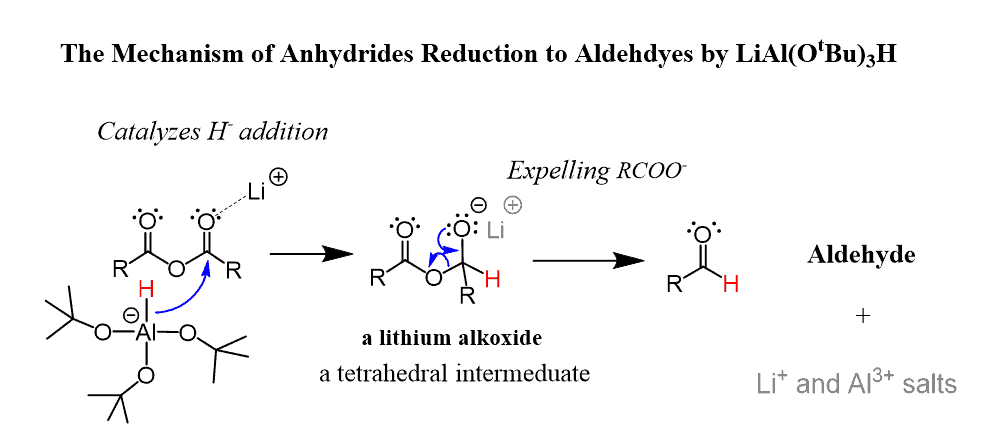
To stop the reduction at the aldehyde, a less powerful reducing agent such as lithium tri(t-butoxy) aluminum hydride LiAl(OtBu)3H can be used:
Check Also
- Preparation of Carboxylic Acids
- Naming Carboxylic Acids
- Naming Nitriles
- Naming Esters
- Naming Carboxylic Acid Derivatives – Practice Problems
- The Addition-Elimination Mechanism
- Fischer Esterification
- Ester Hydrolysis by Acid and Base-Catalyzed Hydrolysis
- What is Transesterification?
- Esters Reaction with Amines – The Aminolysis Mechanism
- Ester Reactions Summary and Practice Problems
- Preparation of Acyl (Acid) Chlorides (ROCl)
- Reactions of Acid Chlorides (ROCl) with Nucleophiles
- R2CuLi Organocuprates – Gilman Reagent
- Reaction of Acyl Chlorides with Grignard and Gilman (Organocuprate) Reagents
- Reduction of Acyl Chlorides by LiAlH4, NaBH4, and LiAl(OtBu)3H
- Reduction of Carboxylic Acids and Their Derivatives
- Preparation and Reaction Mechanism of Carboxylic Anhydrides
- Amides – Structure and Reactivity
- Naming Amides
- Amides Hydrolysis: Acid and Base-Catalyzed Mechanism
- Amide Dehydration Mechanism by SOCl2, POCl3, and P2O5
- Amide Reduction Mechanism by LiAlH4
- Reduction of Amides to Amines and Aldehydes
- Amides Preparation and Reactions Summary
- Amides from Carboxylic Acids-DCC and EDC Coupling
- The Mechanism of Nitrile Hydrolysis To Carboxylic Acid
- Nitrile Reduction Mechanism with LiAlH4 and DIBAL to Amine or Aldehyde
- The Mechanism of Grignard and Organolithium Reactions with Nitriles
- The Reactions of Nitriles
- Converting Nitriles to Amides
- Carboxylic Acids to Ketones
- Esters to Ketones
- Carboxylic Acids and Their Derivatives Practice Problems
- Carboxylic Acids and Their Derivatives Quiz
- Reactions Map of Carboxylic Acid Derivatives
