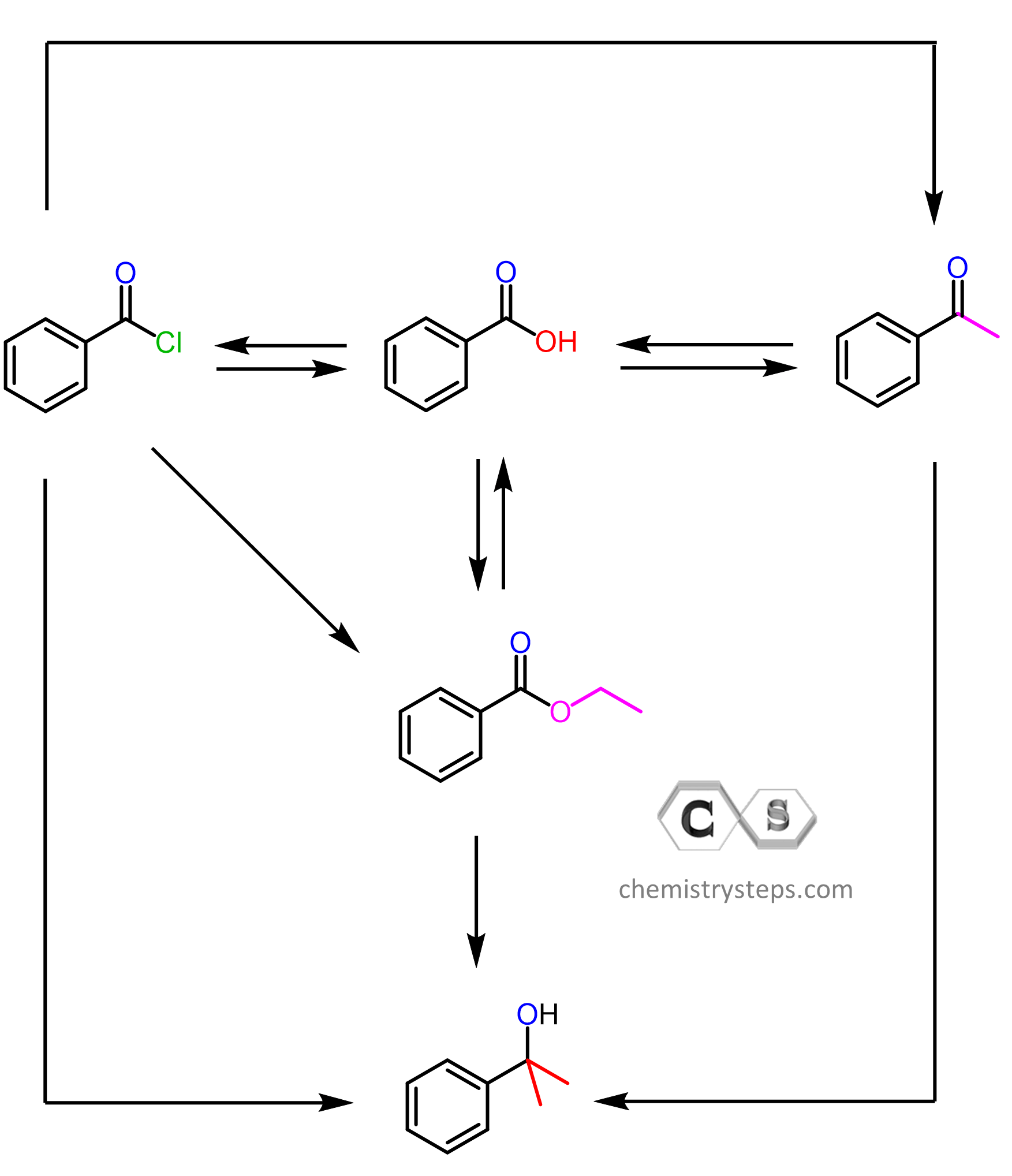
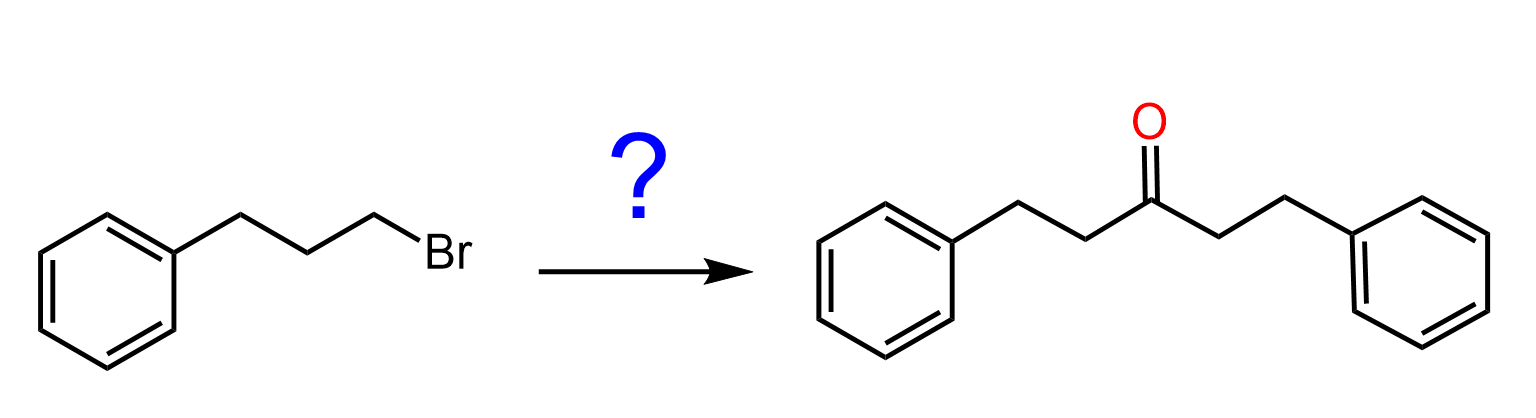
Gilman reagents are organocuprates with a general formula of R2CuLi. To be more specific, these are lithium dialkylcuprates. You can look at them as “weaker” Grignard or organolithium reagents, meaning they are less reactive but also unique in a few important ways. Like any organometallics we have learnt so far, we treat them as a source of an R–.
So, what does a less reactive organometallic mean in practice when comparing the reactions of Gilman reagents with those of Grignard reagents? For starters, remember that R2CuLi organocuprates are generally unreactive towards aldehydes, ketones, carboxylic acids, and esters. While, we do not need to worry about esters, to restrict the reactions with aldehydes and ketones, they are carried out at -78 oC to – 40 oC.
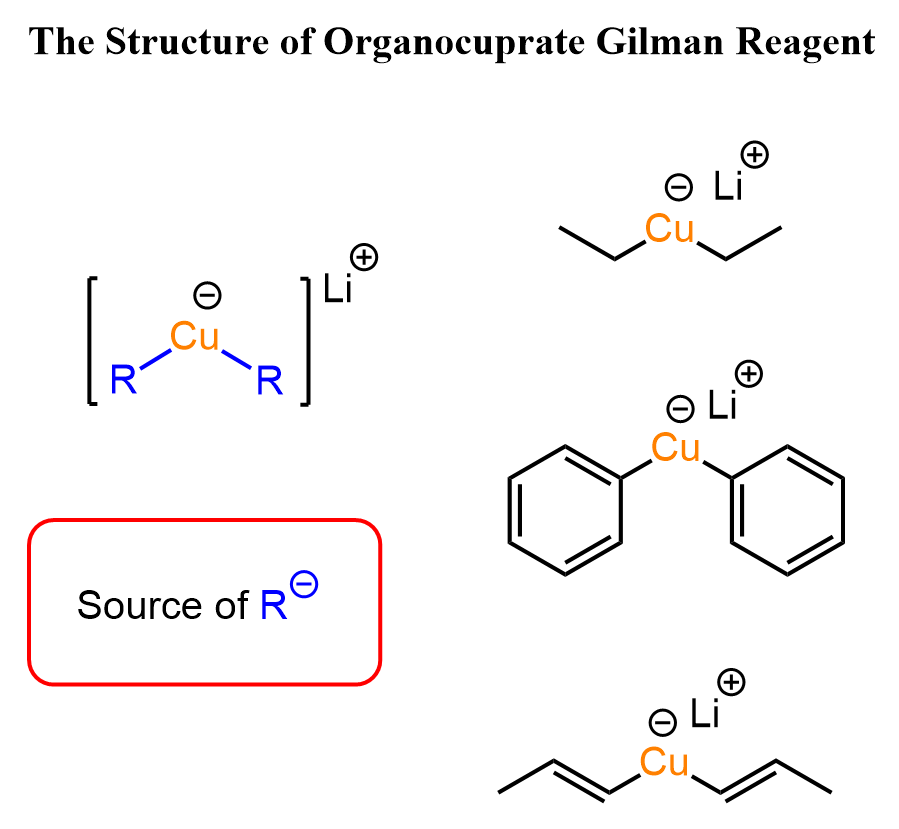
The only carbonyls they readily add to at these temperatures are acid chlorides, as those are the most reactive carboxylic acid derivatives. Importantly, they do only one nucleophilic addition, and as a result, the product of this reaction is a ketone.

The selective reactivity of Gilman reagents to acid chlorides is especially pronounced at lower temperatures (-78 oC), and the ROCl moiety of a molecule containing another carbonyl group can be selectively converted into a ketone:
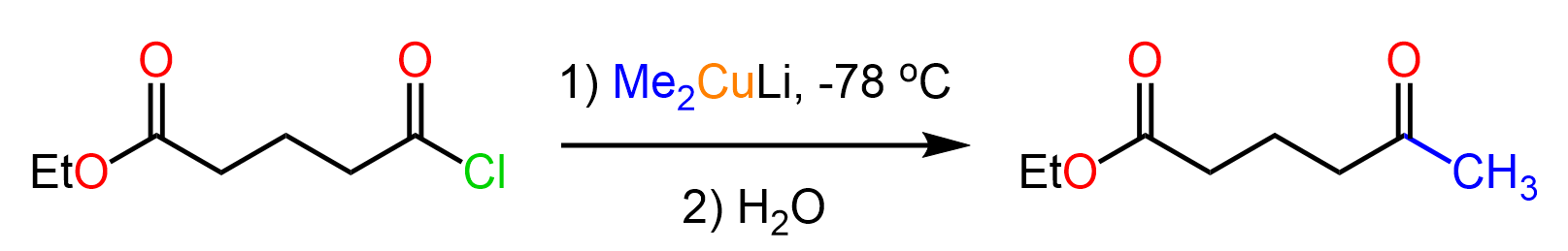
The mechanism is identical to what we saw in the reaction of Grignard reagents with carbonyl compounds. The nucleophilic addition to the carbonyl forms a tetrahedral intermediate, which is then hydrolyzed to a ketone:

Recall that the addition of Grignard reagents to acid chlorides cannot be stopped at the intermediate ketone; therefore, a tertiary alcohol is produced.
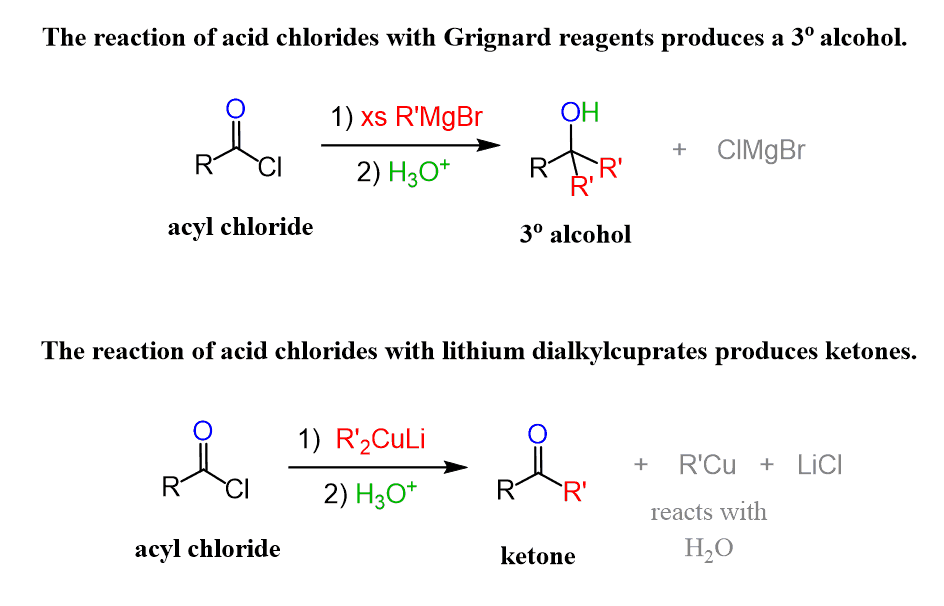
The reason for this difference is that the Grignard reagent is more reactive, and as soon as the ketone intermediate is formed, it attacks the carbonyl again, forming a tertiary alcohol. In the case of organocuprates, the tetrahedral intermediate that is formed after the first addition to the carbonyl is believed to be more stable; it transforms to a ketone upon the aqueous workup. Additionally, the organocuprate itself is not as reactive to attack the ketone, especially if the acid chloride is still present in the solution.
The Conjugate Addition of Organocuprates
Another important difference between Grignard and organolithium reagents is the reaction of organocuprates with α, β-unsaturated carbonyl compounds. Unlike Grignard reagents, the nucleophilic carbon attacks the C=C double bond instead of the C=O carbon:
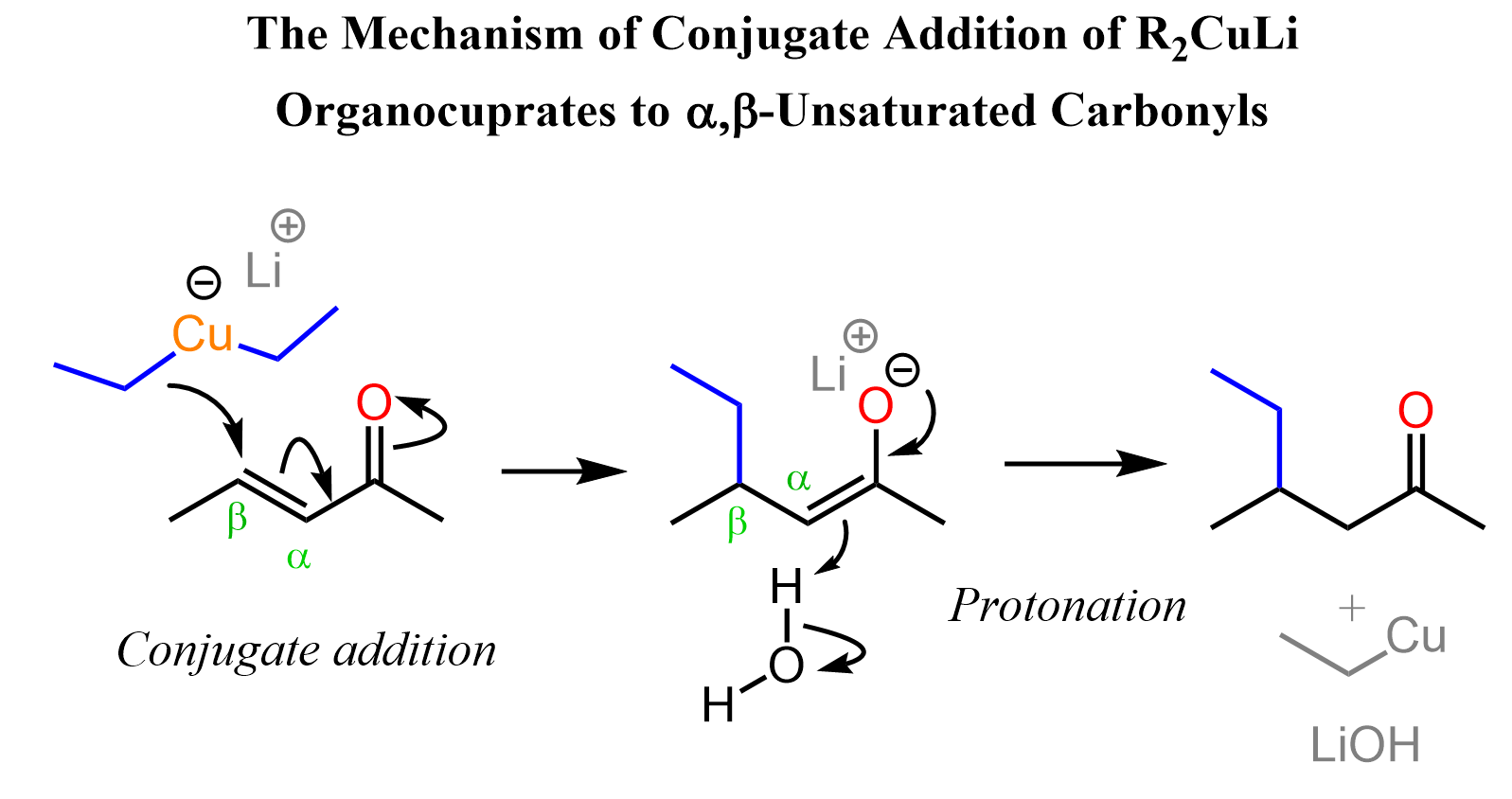
Notice that the addition is facilitated by the carbonyl group, which is converted into an enolate as the electrons are pushed to oxygen. The enolate is then hydrolyzed to regenerate the carbonyl, and the overall reaction looks like a simple addition to the C=C double bond:

This is called a conjugate addition, and overall it is a 1,4-addition, which we can see by numbering the atoms from the oxygen to the farthest C=C carbon. Recall here too that Grignard and organolithium reagents add to the carbonyl carbon, and we classify them as 1,2-additions:

One way to explain the addition to the C=C double bond is by drawing the resonance structure of the α, β-unsaturated carbonyls, where the terminal carbon of the C=C bond bears a positive charge. This means the carbon is electron-deficient, which makes it reactive towards the nucleophilic carbon of the Gilman reagent:

Gilman reagent is not the only nucleophile that does 1,4-additions to unsaturated compounds. It is a characteristic reaction of relatively weak nucleophiles such as halides, cyanide ion, thiols, alcohol, and amines:

Overall, these are called Michael addition reactions, and we discussed them in more detail here.
Double Alkylation in Conjugate Addition of Organocuprates
You may encounter a problem asking you to put two alkyl groups in a Michel acceptor:
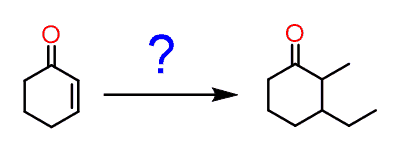
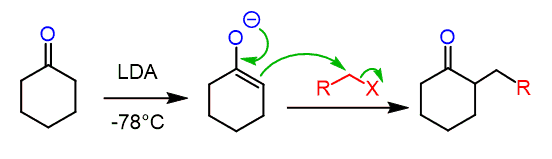
And this may look unclear since you know that using a Gilman reagent would alkylate the β position, giving a carbonyl which is difficult to selectively alkylate on the target position:
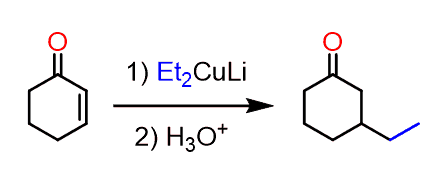
None of the sides is less substituted to make the use of LDA or NaH beneficial for a regioselective alkylation.
So, the trick here is to add an alkyl halide before the acidic workup. In this case, the intermediate enolate is still reactive, and the allylation occurs on the side where the organocuprate had reacted:
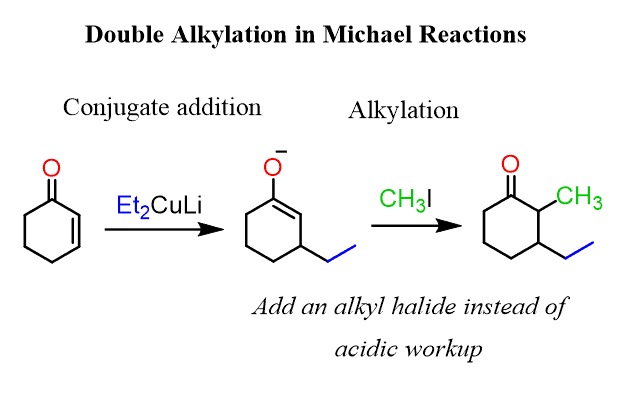
The second alkylation that occurs on the enolate intermediate occurs as the enolate is a great nucleophile, thus attacking the methyl iodide or any alkyl halide added to the reaction.
For more details about the alkylation of enolases, check the separate post here.
The Coupling Reactions of Gilman Reagents
Gilman reagents can be reacted with organohalides such as methyl, primary, aryl, and vinyl halides to connect the two carbon segments of the reagents. These types of reactions are called coupling reactions, and this particular one is the Corey-Posner/Whitesides-House reaction:
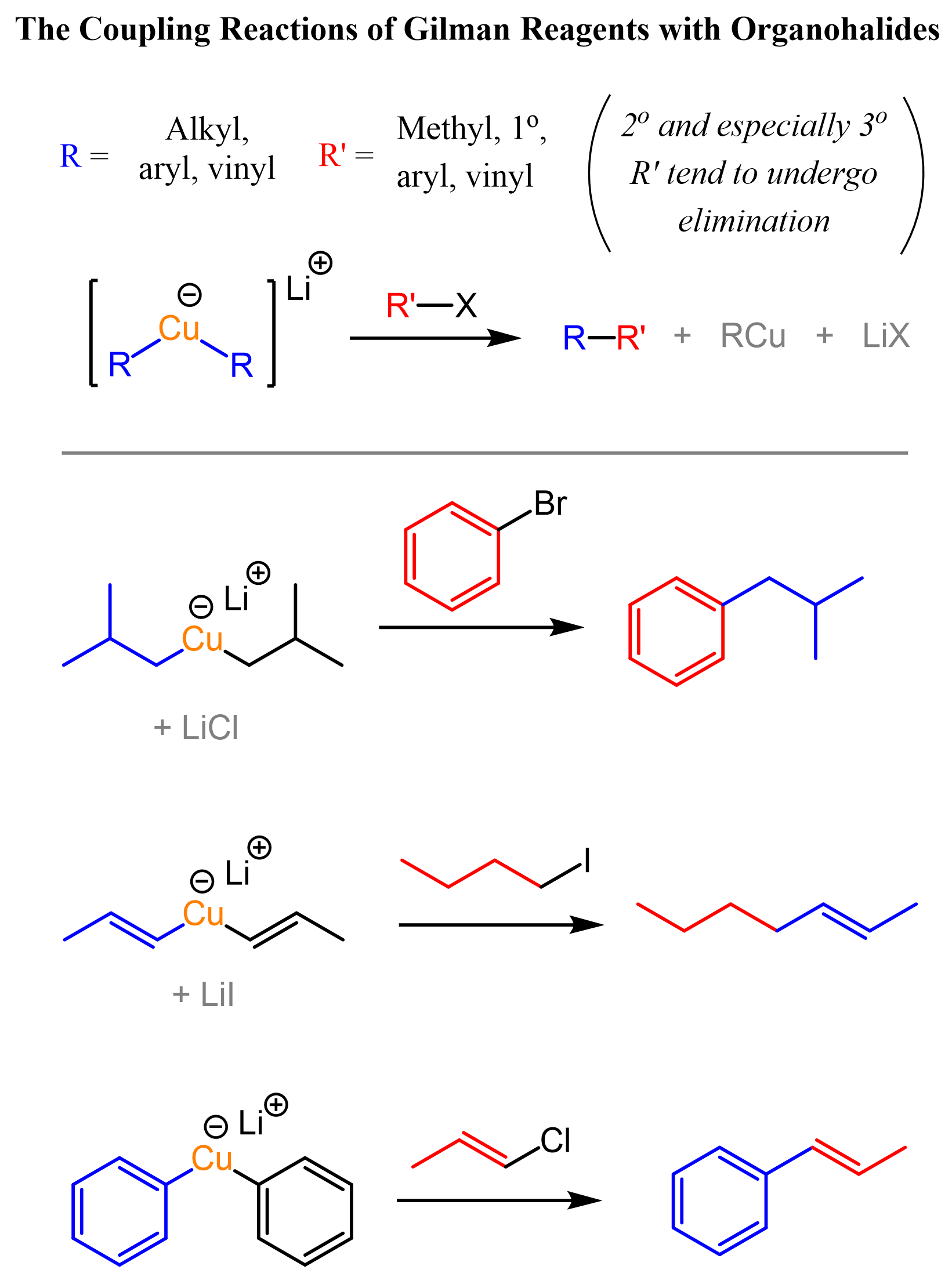
Like in traditional SN2 and E2 competition, secondary and especially tertiary alkyl halides are prone to E2 elimination. This suggests an ionic mechanism for the coupling of organocuprates with organohalides; however, there are other factors supporting the radical pathway, and it is a subject of debate and research. Overall, just keep in mind that even though we may use curved arrows to show the mechanism of organocuprate reactions, it is still not well understood how they occur.
The Reaction of Gilman Reagents with Epoxides
Gilman reagents are great nucleophiles in SN2-type reactions, and they attack epoxides at the less-substituted carbon atom. Overall, the addition of Gilman reagents to epoxides is a two-step process through which an alcohol is formed. In the first step, the epoxide ring is attacked at the least substituted carbon atom, forming an alkoxide intermediate, which is then treated with water or acidic workup to give the final product alcohol:

This, by the way, is similar to how Grinard and organolithium reagents react with epoxides.
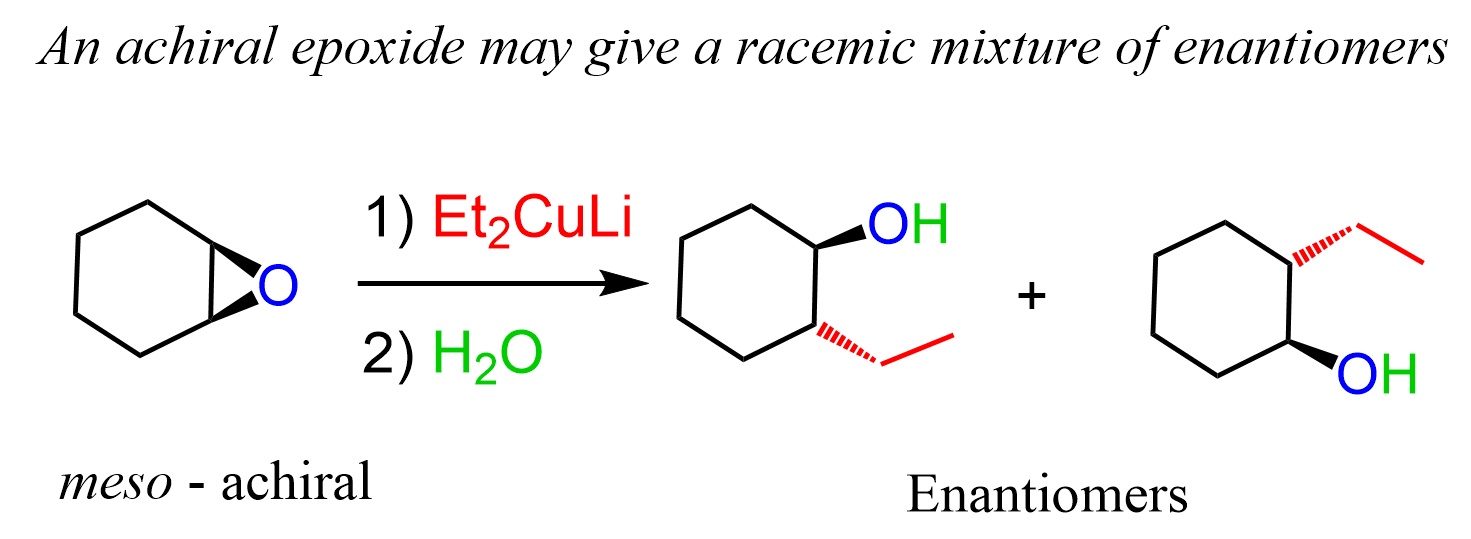
Notice that if the achiral epoxide produces an achiral product, whereas a chiral epoxide produces the given enantiomer of the product. Meso epoxides produce a racemic mixture of enantiomers because the symmetry of the molecule is broken and the chirality centers of the meso epoxide become “relevant”. Remember, meso compounds contain chirality centers, but the molecule is achiral because of an internal symmetry plane:
Preparation of Gilman Reagents
This is normally what we discuss at the beginning of the topic, but I wanted to get straight to the point of what organocuprates are and how they are different from Grignard and organolithium reagents.
The R2CuLi organocuprates are prepared from the corresponding organohalides. We first convert the halide to an organolithium by reacting it with lithium metal. The organolithium is then reacted with a CuX salt in a 2:1 ratio to obtain the organocuprate:
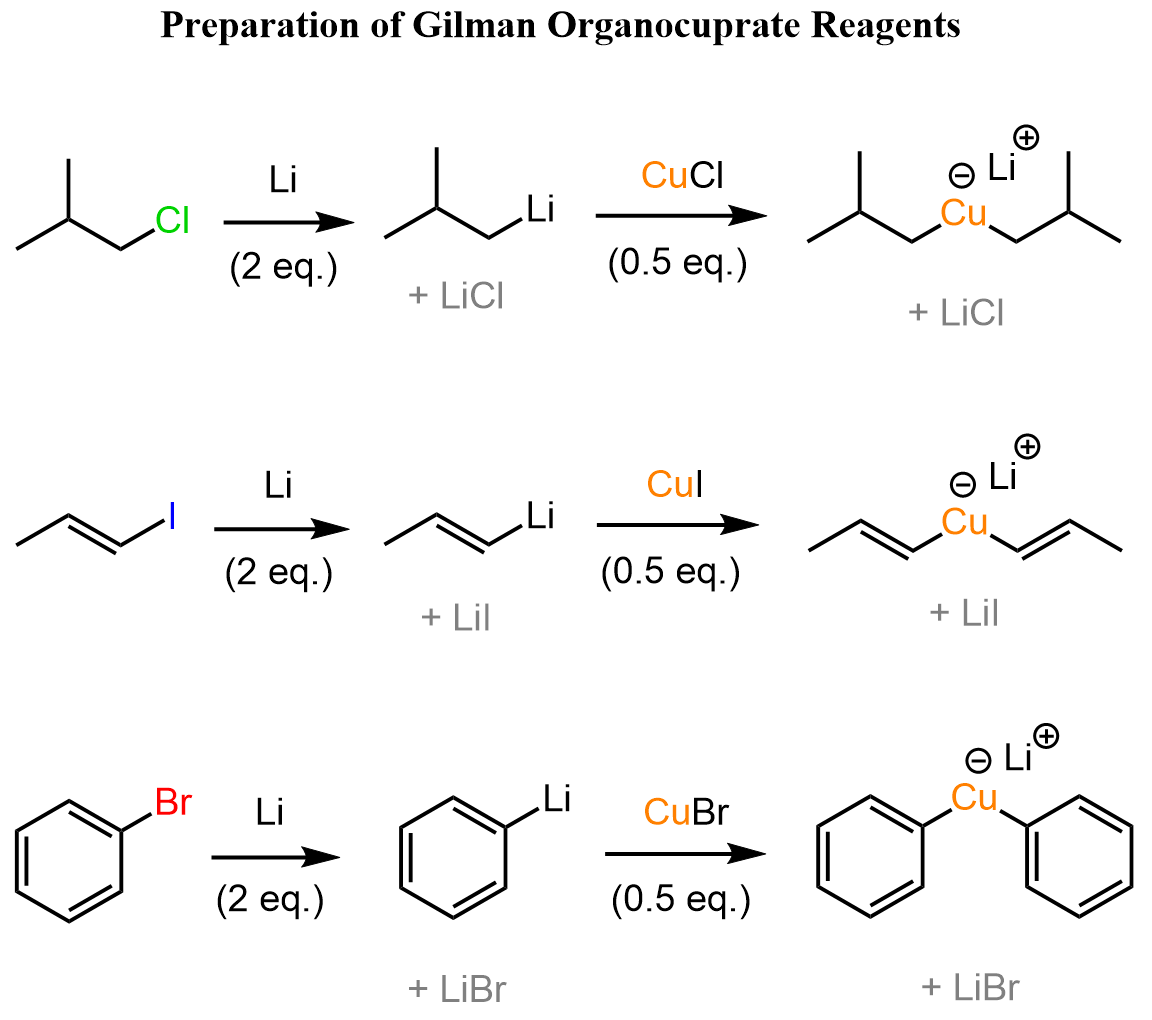
One more thing – the mechanism of organocuprates is a controversial subject, and even though we show it as a “nice” ionic reaction, keep in mind that they may be radical as well. I would say what we showed here is likely an acceptable mechanism for undergraduate courses, especially. Ask your instructor how to go about it.
Check Also
- Preparation of Carboxylic Acids
- Naming Carboxylic Acids
- Naming Nitriles
- Naming Esters
- Naming Carboxylic Acid Derivatives – Practice Problems
- Fischer Esterification
- Ester Hydrolysis by Acid and Base-Catalyzed Hydrolysis
- What is Transesterification?
- Esters Reaction with Amines – The Aminolysis Mechanism
- Ester Reactions Summary and Practice Problems
- Preparation of Acyl (Acid) Chlorides (ROCl)
- Reactions of Acid Chlorides (ROCl) with Nucleophiles
- Reaction of Acyl Chlorides with Grignard and Gilman (Organocuprate) Reagents
- Reduction of Acyl Chlorides by LiAlH4, NaBH4, and LiAl(OtBu)3H
- Preparation and Reaction Mechanism of Carboxylic Anhydrides
- Amides – Structure and Reactivity
- Naming Amides
- Amides Hydrolysis: Acid and Base-Catalyzed Mechanism
- Amide Dehydration Mechanism by SOCl2, POCl3, and P2O5
- Amide Reduction Mechanism by LiAlH4
- Reduction of Amides to Amines and Aldehydes
- Amides Preparation and Reactions Summary
- Amides from Carboxylic Acids-DCC and EDC Coupling
- The Mechanism of Nitrile Hydrolysis To Carboxylic Acid
- Nitrile Reduction Mechanism with LiAlH4 and DIBAL to Amine or Aldehyde
- The Mechanism of Grignard and Organolithium Reactions with Nitriles
- The Reactions of Nitriles
- Converting Nitriles to Amides
- Carboxylic Acids to Ketones
- Esters to Ketones
- Carboxylic Acids and Their Derivatives Practice Problems
- Carboxylic Acids and Their Derivatives Quiz
- Reactions Map of Carboxylic Acid Derivatives