The addition of cyanide ion to the carbonyl group is another common reaction of aldehydes and ketones. The product of these reactions is a cyanohydrin.
It works in a slightly basic conditions since the HNC itself does not dissociate much to produce the –CN nucleophile in enough concentration:

Hydrocyanic acid will react with the carbonyl establishing an equilibrium over the time, however, best yields are obtained when a salt of a cyanide ion such as KCN or NaCN is used instead of the HCN:

The mechanism of –CN Addition to Aldehydes and Ketones
When a cyanide salt is used, the reaction starts with a nucleophilic addition of the –CN to the carbonyl forming a negatively charged intermediate – an alkoxide ion. An acidic workup using HCN or other acids forms the cyanohydrin:

One thing to keep in mind when working with cyanides is their high toxicity and hydrocyanic acid is especially dangerous since it is also volatile.
If, however, HCN is used, it is first converted into cyanide ion by catalytic amount of base after which the reaction proceeds as shown above.
Cyanohydrins in Organic Synthesis
There is one key difference in the reaction of aldehydes and ketones with cyanide compared to the ones with water, alcohols, and amines. And that is the formation of a new carbon-carbon bond.
This is a great feature since it achieves what organometallics such as the Grignard reagent do without the need for strong basic conditions.
How is the formation of cyanohydrin comparable to organometallics, right?
Well, cyanohydrins are useful intermediates in the synthesis of other functional groups.
For example, they can be converted to ɑ-hydroxy acids by acid-catalyzed hydrolysis:
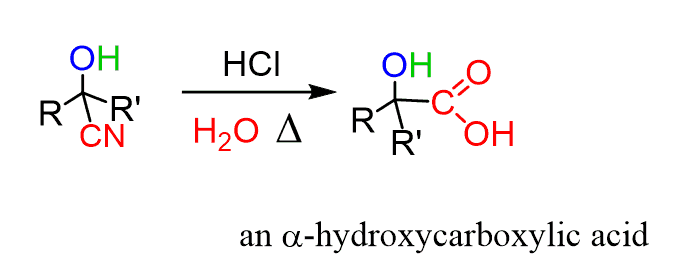
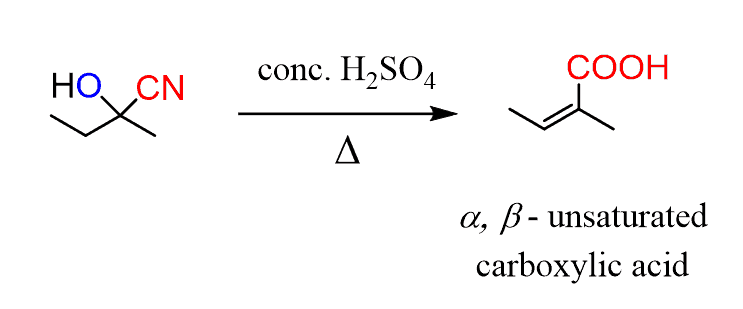
A concentrated sulfuric acid, on the other hand, can convert the cyanohydrin to a α, β-unsaturated acids. So, two things are happening here, first is the hydrolysis of the cyano group and second, a dehydration of the alcohol:
The cyanide group can be reduced to an amine with LiAlH4 or forming a β-aminoalcohol:
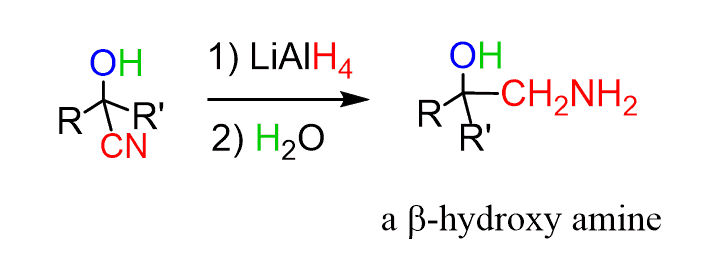
And finally, in basic solutions, the cyanohydrin will be converted back to the carbonyl compound. The alkoxy ion, formed by the deprotonation of the OH group, kicks out the cyano group since it is a weaker base [pKa (HCN) = 9.2] than the hydroxide ion:

Check Also:
- How to Name a Compound with Multiple Functional Groups
- Preparation of Aldehydes and Ketones
- Nucleophilic Addition to Carbonyl Groups
- Reactions of Aldehydes and Ketones with Water
- Reactions of Aldehydes and Ketones with Alcohols: Acetals and Hemiacetals
- Acetals as Protecting Groups for Aldehydes and Ketones
- Formation and Reactions of Imines and Enamines
- Reductive Amination
- Acetal Hydrolysis Mechanism
- Imine and Enamine Hydrolysis Mechanism
- Hydrolysis of Acetals, Imines, and Enamines-Practice Problems
- Grignard Reaction with Practice Problems
- Grignard Reaction in Organic Synthesis with Practice Problems
- The Wittig Reaction: Examples and Mechanism
- The Wittig Reaction: Practice Problems
- Aldehydes and Ketones to Carboxylic Acids
- Reactions of Aldehydes and Ketones – Practice Problems
- Aldehydes and Ketones Reactions Practice Quiz
- Reactions Map of Aldehydes
- Reactions Map of Ketones

Hi! I might be missing something, but I think there is an error in the last paragraph.
It says that:
“in basic solutions, the cyanohydrin will be converted back to the carbonyl compound. The alkoxy ion formed by the nucleophilic addition of –OH kicks out the cyano group”
but, in the mechanism shown below that, we can see that the alkoxy ion is formed by the deprotonation with -OH (base).
Thanks for all your work!
Hi there,
Thanks for letting me know! Fixed.