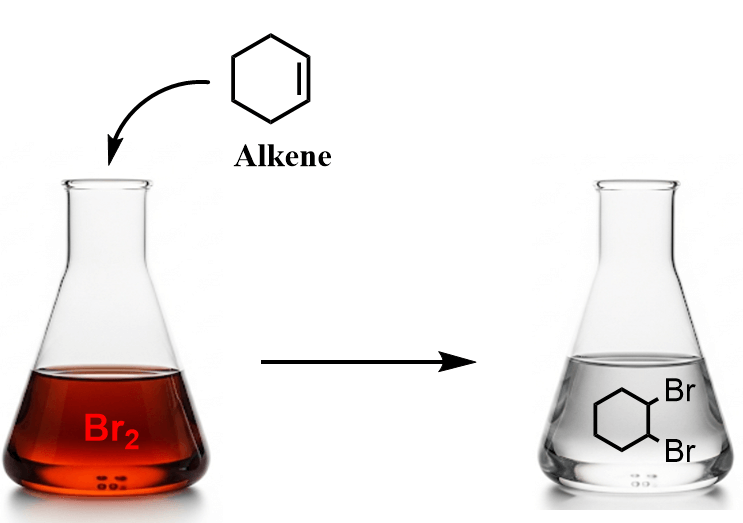
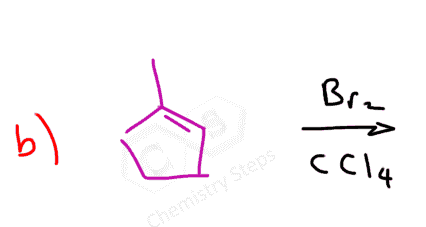Alkenes react at low temperatures with either pure liquid bromine or a bromine solution in an organic solvent such as tetrachloromethane. During the reaction, the double bond breaks, and each carbon atom gains a bromine atom, forming a vicinal dibromide:

What’s particularly interesting about the reaction of alkenes with bromine is that it serves as a simple and effective test for the presence of a double bond. The reddish-brown color of bromine disappears as a colorless dibromo compound is formed:
Chlorine reacts the same way with alkenes, forming chloronium ion, which, in general, is called halonium ion. F2 and I2 are not synthetically useful for this reaction as F2 reacts explosively with the alkene, while the reaction with I2 does not proceed to a significant extent:

The Mechanism of Alkenes’ Reaction with Bromine
The reaction is a two-step process. In the first step, the pi electrons of the double attack one of the Br atoms, polarizing the Br-Br bond and eventually breaking it by forming a three-membered ring intermediate known as a bromonium ion. In a more general terminology, these are called halonium ions.
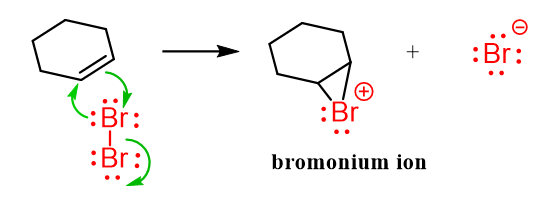
This may look strange because we don’t see a nucleophile attacking a bromine molecule every day. However, remember that double bonds are electron-rich because their double bonds contain an extra pair of electrons compared to single bonds. These extra electrons are found in the π bond, which is weaker and more reactive than a σ bond.
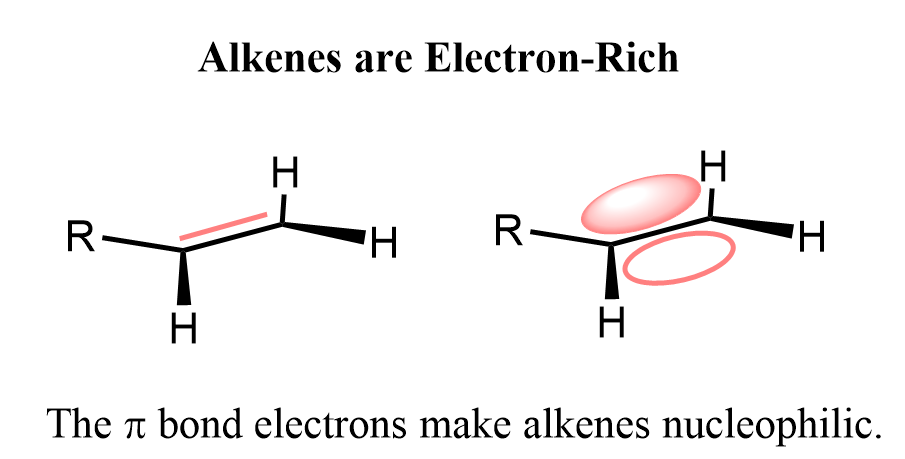
We have talked more about the nucleophilicity of the double bonds and why the reactions of alkenes are called electrophilic addition reactions here, so feel free to check that out as well.
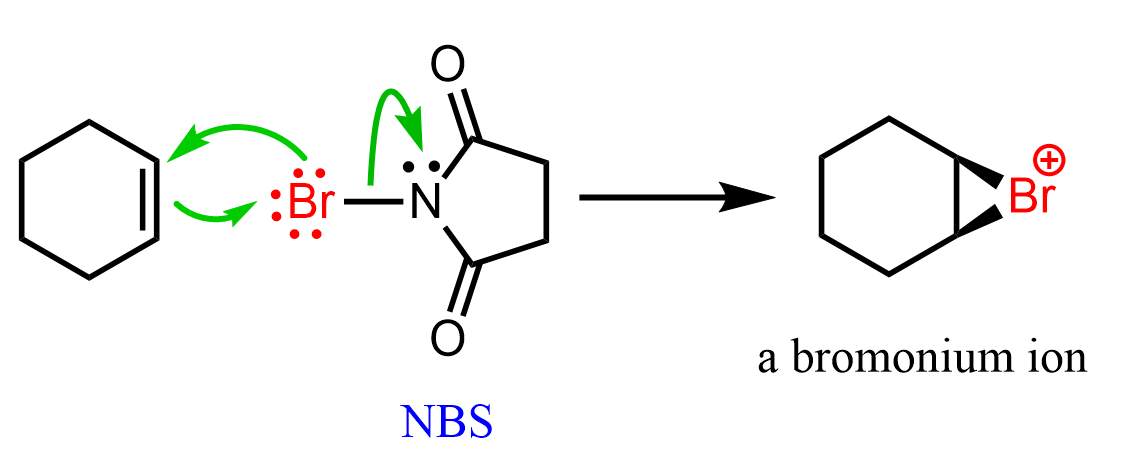
Although today’s focus is specifically on the reaction of alkenes with bromine, it is worth mentioning that halonium ions can also be prepared by using N-bromosuccinimide (NBS) instead of molecular bromine:
The Reactivity of Bromonium Ion
What do we notice about the halonium ion? It is a three-membered ring, and they are also charged. Three-membered rings are associated with high ring strain, and charged species are not particularly stable in organic chemistry.

If you have already covered epoxides, recall their reactivity towards almost any nucleophile due to their great ring strain:

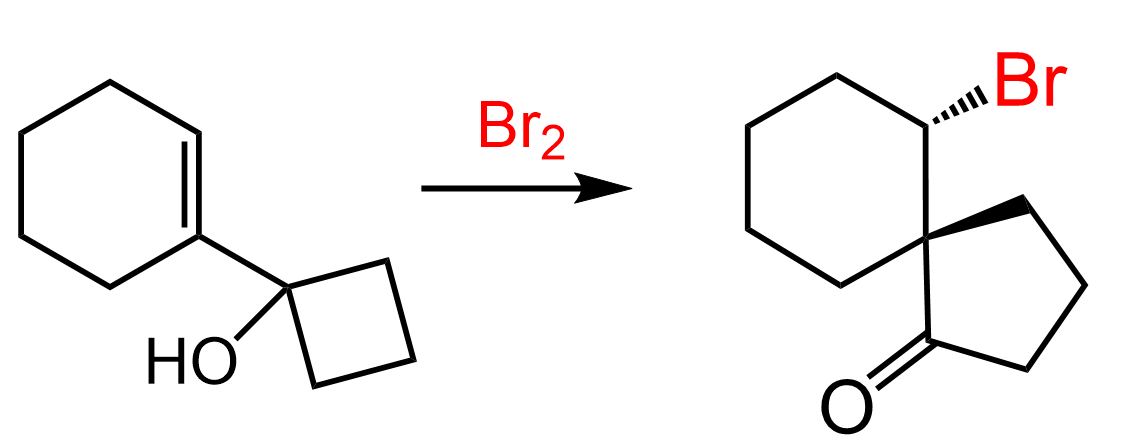
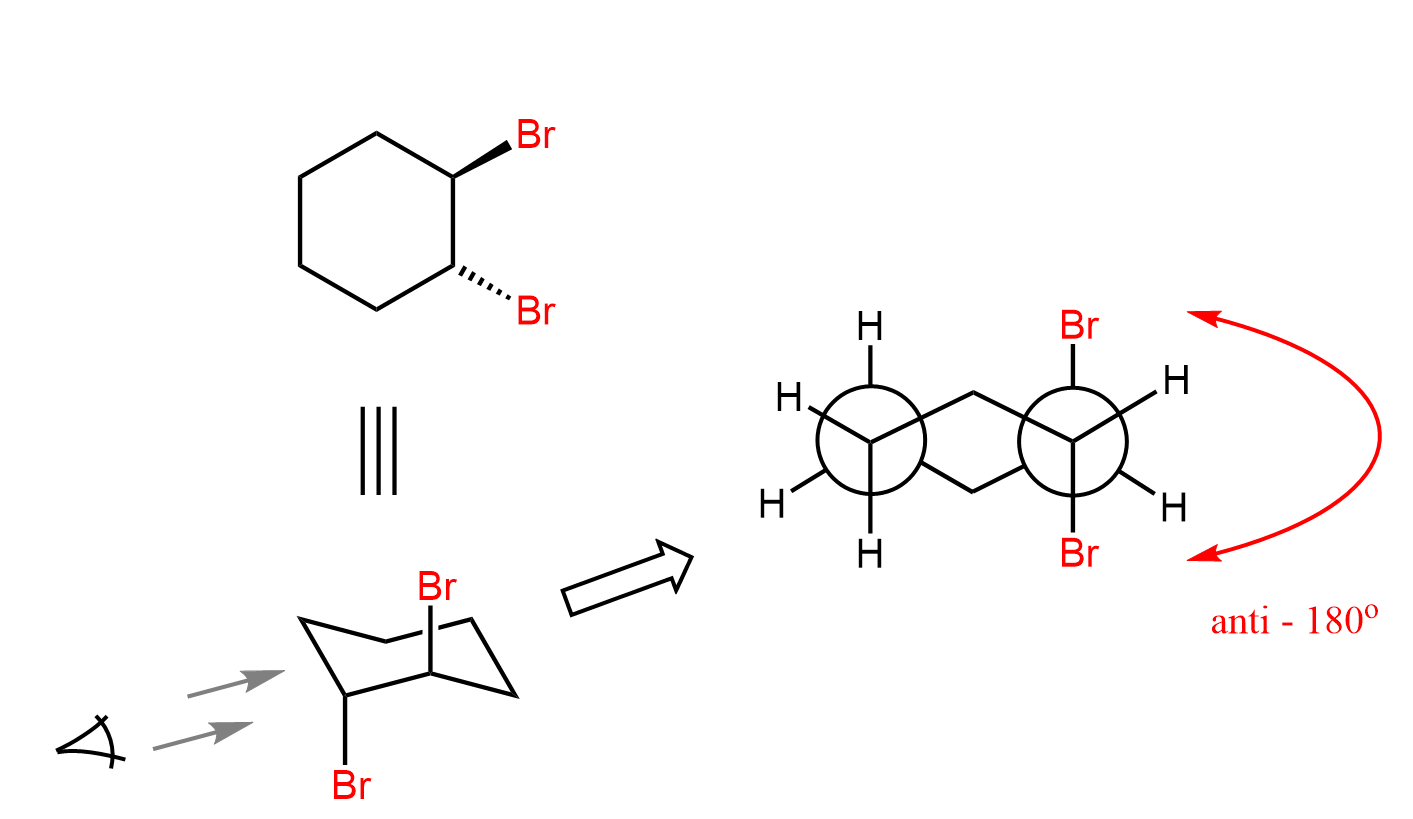
Similar to epoxides, the bromonium ion is also very reactive and is attacked by any nucleophile present in the solution. Now, is there a nucleophile present in the solution? Organic solvents such as carbon tetrachloride are inert, and that’s exactly why we choose them. We’ll later see how the solvent affects this reaction, but for now, let’s recall that when the Br–Br bond was broken, a bromide ion (Br⁻) was formed alongside the bromonium ion. Bromide is quite a good nucleophile, and being in such proximity, it quickly attacks the bromonium ion, breaking one of the C–Br bonds and relieving the ring strain:

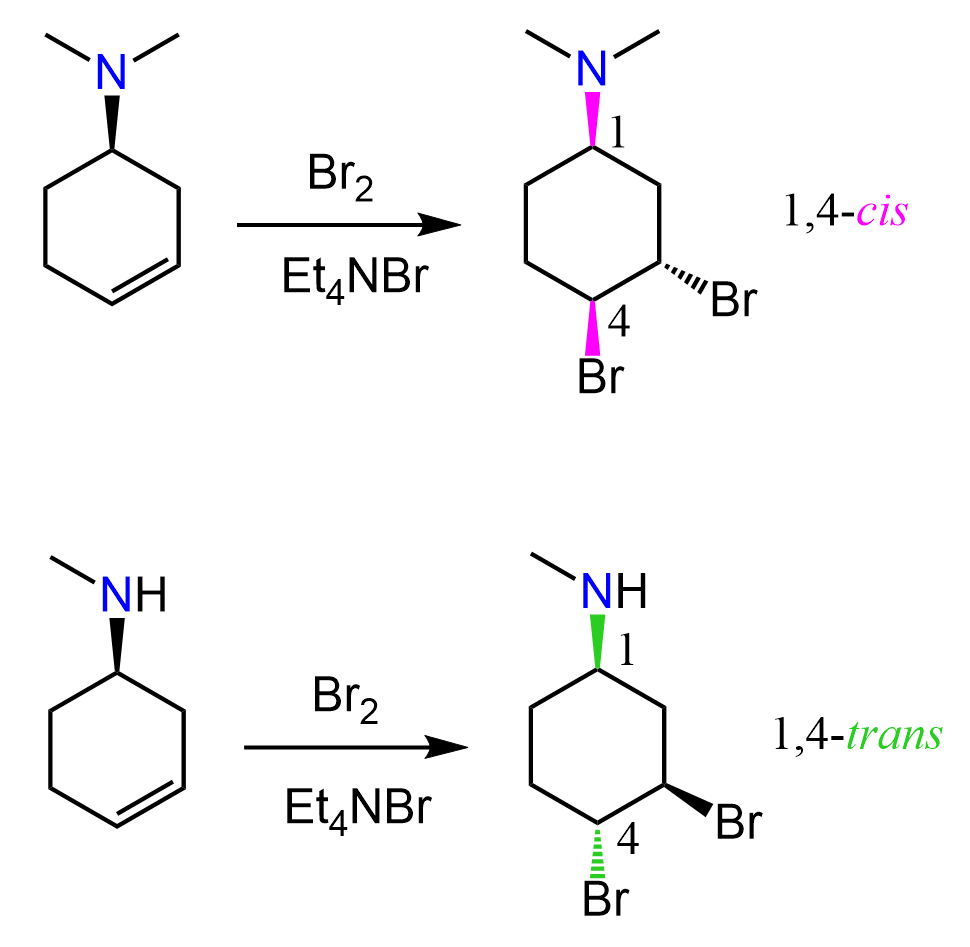
Notice that the bromine in the bromonium ion is the leaving group, and the bromide ion is the nucleophile, and overall, we have an SN2 substitution where the two species are at 180o. As a result, the two bromines have an anti orientation in the ring. Recall from Newman projections that anti conformation is when two groups of interest are anti-periplanar, i.e., the dihedral angle between them is 180o:

We can also draw the Newman projection of this cyclohexane’s chair conformation to see the anti orientation of the two bromines:
Bromination of Alkenes in Water
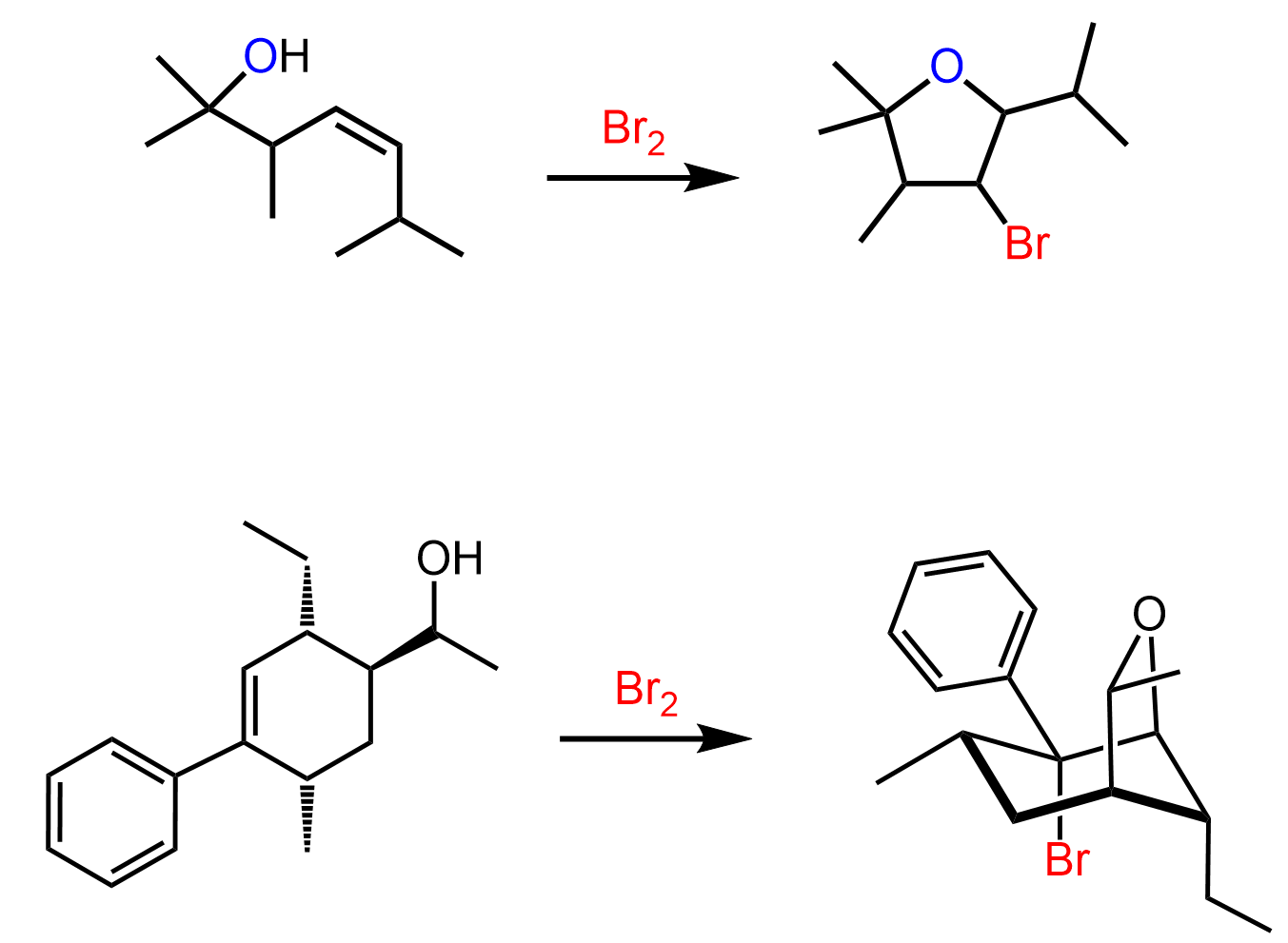
Although water is a weak nucleophile, it can outcompete the bromide ion in opening the bromonium ion due to its high concentration. This leads to the formation of a product called a halohydrin, which contains both a halogen and a hydroxyl group on adjacent carbons.
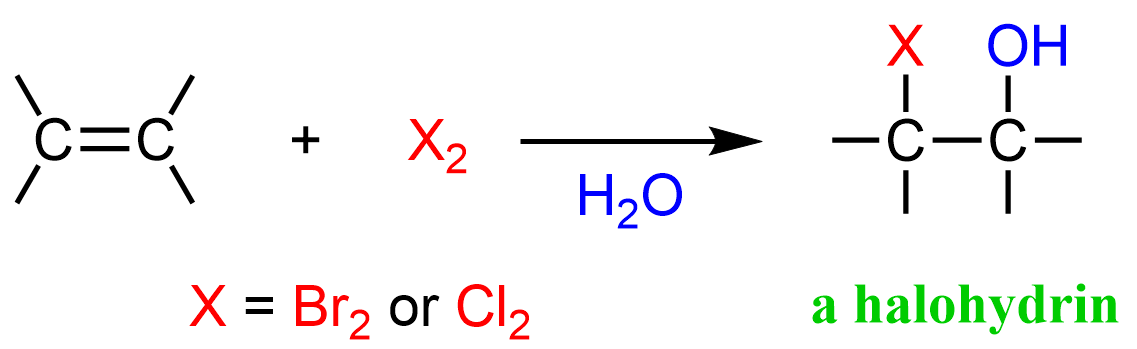
When an unsymmetrical alkene is used, the nucleophilic attack by water occurs at the more substituted carbon of the bromonium ion, and the halogen ends up on the less substituted carbon.

This is explained by the stability of the energy differences between the two possible transition states:
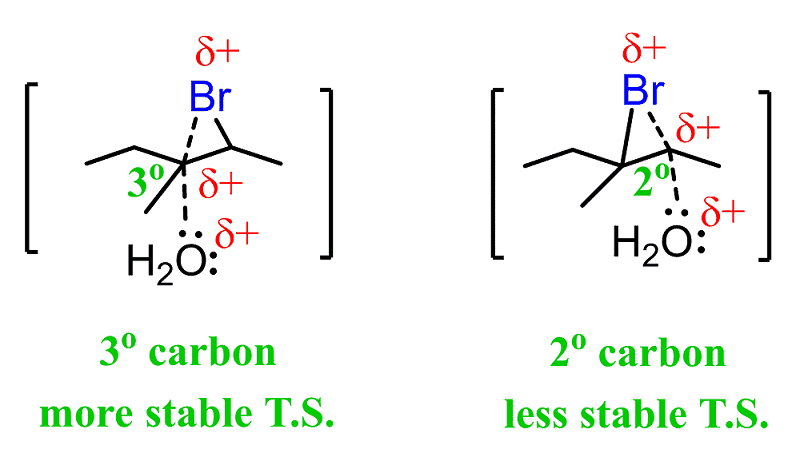
The first transition state has a partial positive charge on a more substituted carbon, making it more stable.
The Stereochemistry of Halohydrin Formation
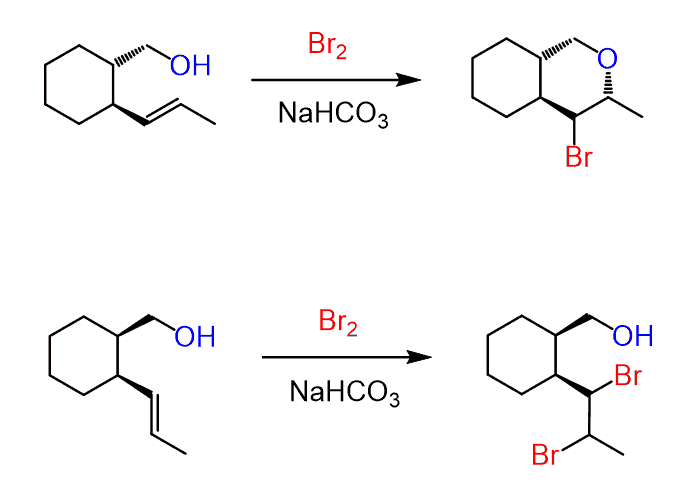
Notice that in the reaction above, a pair of enantiomeric halohydrins is formed. This is the most common outcome you will see when unsymmetrical alkenes react with bromine in the presence of water.
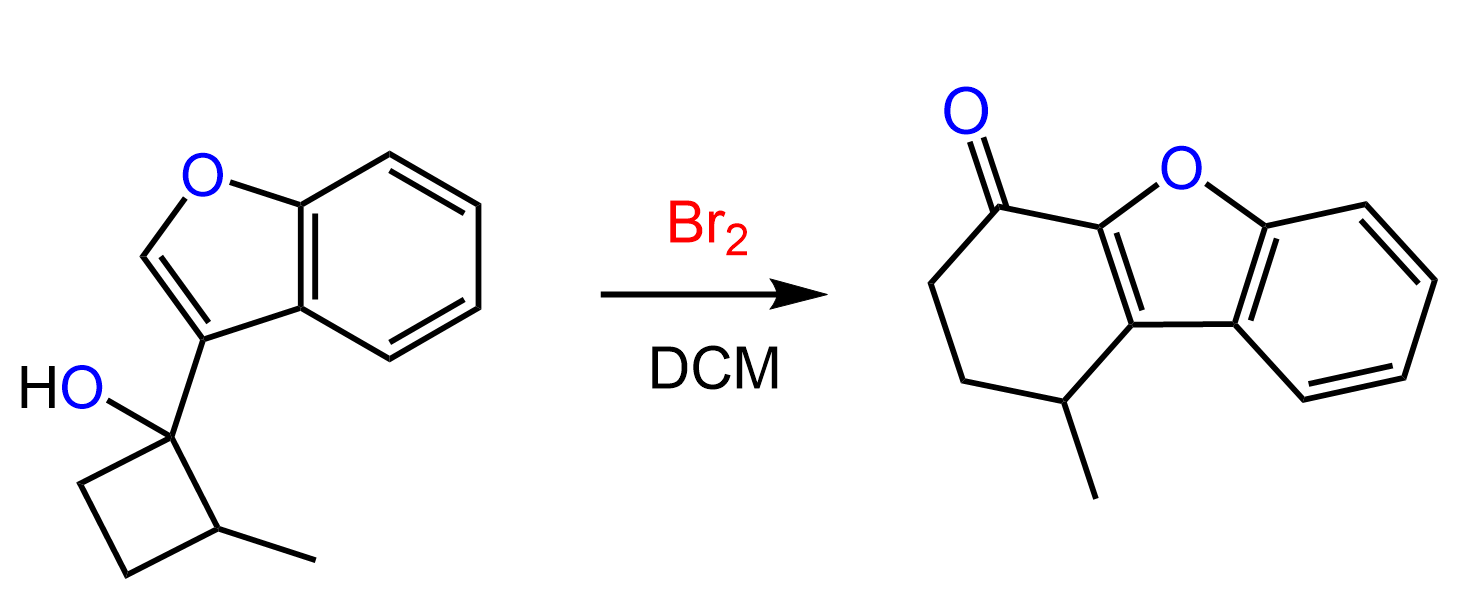
It is also possible to obtain a pair of diastereomers when there is another chiral center in the alkene that is not part of the reaction. For example, here is how the reaction proceeds when water is present in large quantities:
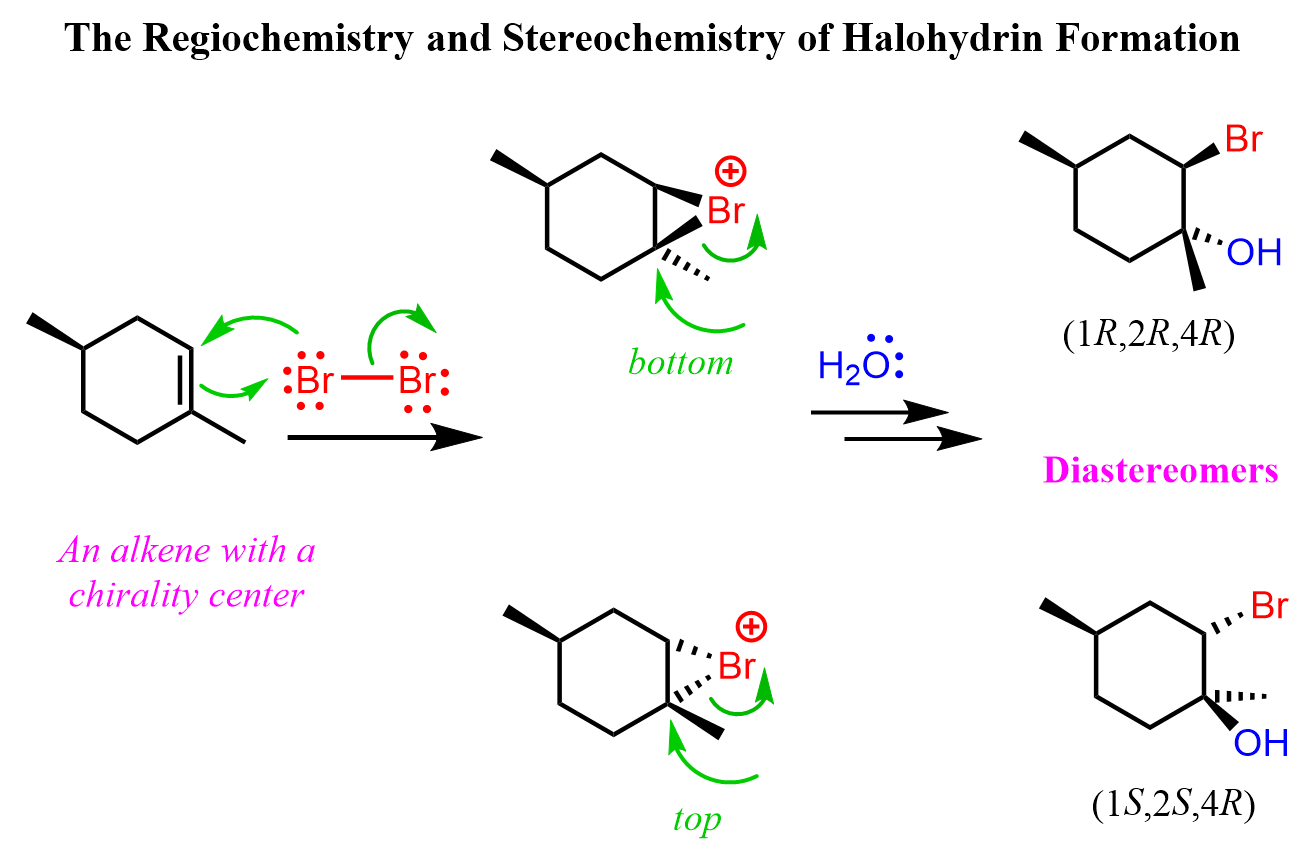
Notice that the chiral center in the starting material is not involved in the reaction, and the addition of water to both faces of the carbocation leads to the formation of diastereomers. Typically, in this type of reaction, when the starting material is achiral, the product is a racemic mixture of enantiomers.
Other Nucleophiles in Bromination of Alkenes
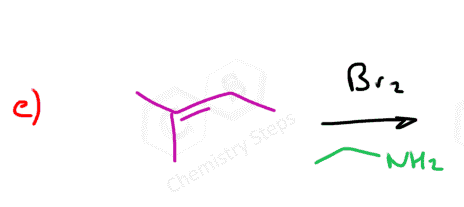
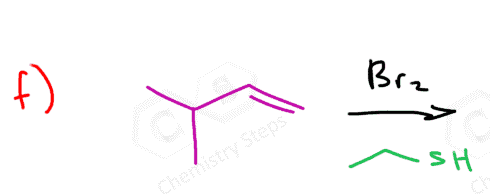
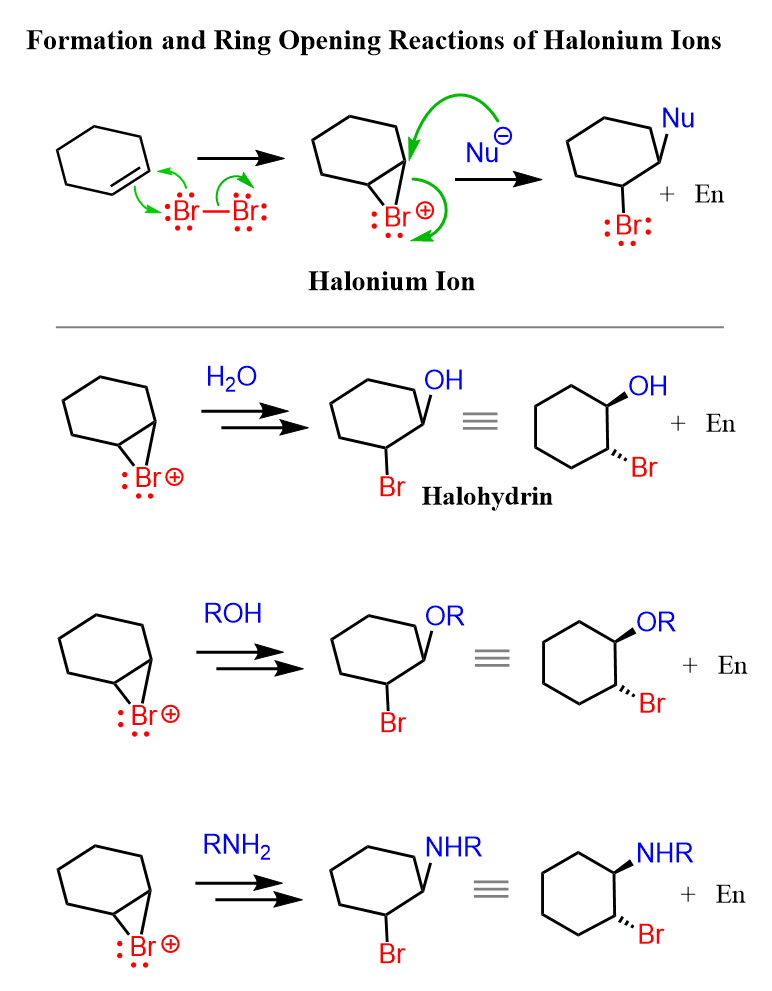
Instead of water, we can also use other nucleophiles such as alcohols, thiols, and amines in the bromination of alkenes. These nucleophiles behave similarly to water in that they attack the bromonium ion formed during the reaction. For example, if an alcohol is present in the reaction mixture, it can open the bromonium ion to form a bromoether instead of a bromohydrin. Likewise, thiols and amines can lead to the formation of bromothioethers or bromoamines, respectively. The general mechanism remains the same: the alkene reacts with bromine to form the cyclic bromonium ion, and the nucleophile then attacks the more substituted carbon to relieve ring strain.
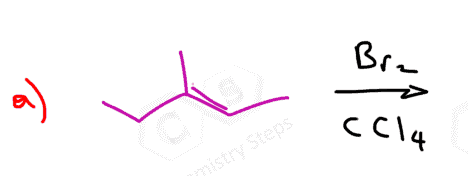
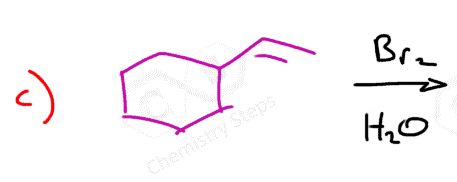
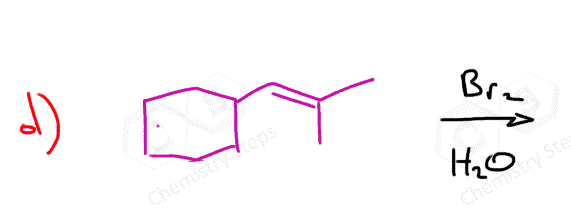
Here are some practice problems on addition reactions of alkenes that proceed through halonium ion intermediates.
We also have separate posts on the halogenation of alkenes and halohydrin formation, so feel free to check those out for more detailed explanations and mechanisms.