Epoxides undergo ring-opening reactions under both acidic and basic conditions. Let’s clarify that by “basic conditions” we refer to a greater class of epoxide ring-openings when they are reacted with good nucleophiles. This includes a number of them such as hydroxides, alkoxides, thiols, cyanides, Grignard reagents, and LiAlH4 etc. In this article, we will focus on the reactions of hydroxides and alkoxides as good nucleophiles together with the ring-openings under acidic conditions.

Let’s first mention what is common in the ring opening of epoxides with good and poor nucleophiles. Notice that the nucleophilic attack occurs from the opposite side of the oxygen because the ring-opening reactions of epoxides proceed by SN2 mechanism.
On the contrary, we can see that the order of the nucleophilic attack and the protonation is reversed. The other important difference is the regiochemistry of these reactions. Notice that good nucleophiles attack the less substituted carbon of the epoxide, while the halide and other poor nucleophiles attack the more substituted carbon atom.
So, what makes these differences in the ring-opening reactions of epoxides under acidic and basic conditions? The differences are explained by both the structure of the epoxide and the reactivity of the nucleophile.
Now, what is it about the structure of epoxides that makes them reactive? The primary cause of the epoxide reactivity is the high ring strain of three-membered rings. Not every three-membered ring can be attacked and opened up by a nucleophile, so the other factor here is the presence of the oxygen which is essentially the leaving group when the nucleophilic attack occurs. Recall though that negatively charged oxygen species are not the best leaving groups, and although epoxides are reactive due to the high ring strain, they still require a good nucleophile to break one of the C-O bonds and open up the three-membered ring. This unfavorable transformation of the neutral oxygen to a negatively charged leaving group is compensated in part by the ring strain of the epoxide and the reactivity of the nucleophile.

The next question is what if the nucleophile is not strong or good enough? This is compensated by turning the oxygen into a good leaving group before the nucleophilic can attack.
Epoxide Ring-Opening Under Acidic Conditions
We mentioned that the strength/reactivity of the nucleophile is the key driving force in the ring-opening of epoxides under basic conditions.
Now, if the nucleophile is not as reactive, then the leaving group must be a lot better than the negatively charged oxygen that we have in the reactions with good nucleophiles. This is achieved by protonating the oxygen in the epoxide because as such, the leaving group is a neutral R-OH rather than a negatively charged alkoxide unit.

Recall the reaction alcohols with acids where the OH group is first protonated before it leaves or is kicked out by the nucleophile:

This is what happens when epoxides are reacted with acids. The oxygen is converted into a good leaving group, after which the halide ion is capable of opening the ring via a nucleophilic attack:

Let’s continue the discussion by addressing some important points in the ring-opening epoxides under acidic conditions.
The Regiochemistry of Epoxide Ring-Opening Under Acidic Conditions
We mentioned that as in the reactions of alcohols with HCl, HBr, or HI hydrohalic acid, the oxygen in the epoxide is first protonated before the nucleophilic attack of the halide ion. What is important to note here is the position that the nucleophile attacks. Under acidic conditions, the nucleophile ends up on the more substituted carbon of unsymmetrical epoxides. Notice that the attack of the bromide ion occurs at the tertiary carbon of the epoxide:

This is a common pattern for the reactions of epoxides with poor nucleophiles. For example, alcohols can also open up the epoxide ring under acidic conditions. In this case, H2SO4 is normally used as the catalyst to avoid any competition with the halide ion of hydrohalic acids.

Notice that the regioselectivity increases with the number of alkyl groups connected to the electrophilic carbon atom. A question to think about here is why in the first place does the nucleophile attack the more substituted carbon and why this selectivity is more pronounced when there is another alkyl group added? This is counterintuitive from the sterics perspective because the alkyl groups block the way of the nucleophile. However, it can be explained by the electronics argument. The epoxide, or more specifically the carbon where the nucleophilic attack occurs in the electrophile, so it must have some electron deficiency, i.e. a partial positive charge. As we know from the stability of carbocations, the more alkyl groups, the more stable they are because of the electron-donation nature of these groups:
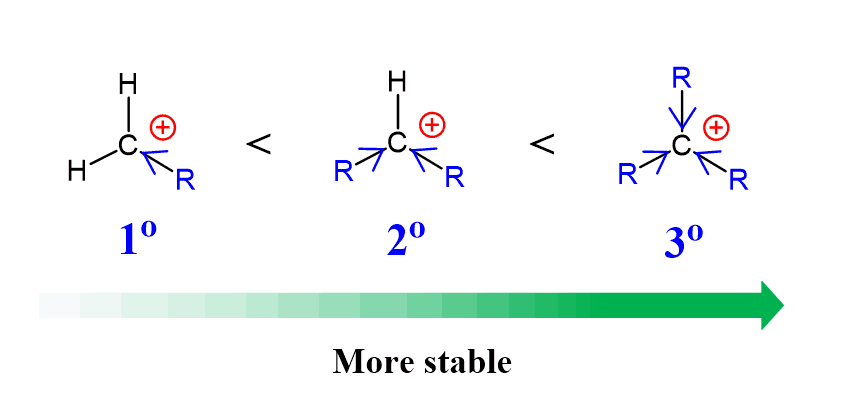
And this is essentially what we have in epoxides: the oxygen is more electronegative and pulls the electron density from the carbons making them electron deficient. The more substituted carbon takes this electron-deficiency as the alkyl groups are stabilizing the partial positive charge. We can also see in the transition state where the bonds are partially broken and the positive charge distribution goes through the more substituted carbon atom:

The same pattern of opening up three-membered rings is observed in the oxymercuration, halogenation, and formation of halohydrins from alkenes:

The structure on the left is the most optimal as the partial positive charge is on the more substituted carbon and this is where the nucleophilic attack occurs.
Reactions of Epoxides with –OH and –OR Nucleophiles
Both hydroxide and alkoxide ions are strong bases and good nucleophiles, therefore, they react with epoxide by direct nucleophilic attack on the less substituted carbon atom. This opens the epoxide ring forming a new alkoxide intermediate which is protonated by aqueous or acidic workup:

The Stereochemistry of Ring Opening with Hydroxide
Achiral Epoxides: The stereochemical outcome of the reaction depends on the structure of the epoxide. Achiral epoxides most often produce achiral products. For example, the following achiral epoxide forms an achiral product when reacted with sodium ethoxide:

Meso Epoxides: Achiral epoxides can also produce a racemic mixture of enantiomers. For example, the cyclohexene epoxide is achiral as it is a meso compound. Remember, meso compounds are achiral because of the internal plane of symmetry, however, they do have chirality centers. When this epoxide is opened up with hydroxide or alkoxide ion, or with any good nucleophile, a racemic mixture of enantiomers is obtained because breaking any of the C-O bonds breaks the symmetry of the molecule as well:

Notice that the carbon that keeps the bond with oxygen, retains the configuration, whereas the one where the nucleophilic attack occurs undergoes an inversion of configuration. This is not always the case, and we’ll address the R and S configuration in ring-opening reactions of epoxides in the next section.
Chiral Epoxides: If the epoxide is chiral, each enantiomer gives the corresponding enantiomeric product. Let’s add a methyl group to one of the carbons, and see how the attack on the less substituted carbon atom produces one enantiomer of the alcohol:

R and S Configuration in Reactions of Epoxides
If only one of the carbons is achiral, it remains achiral in the product too, while the chiral carbon may retain the configuration or may undergo an inversion of configuration. This depends on how the nucleophile affects the priority of the groups on the chiral carbo. In the case of hydroxide and alkoxide addition, the configuration normally does not change as they both bring an oxygen to the chiral carbon. Notice that it was still an oxygen before the reaction, so the configuration does not change. If the nucleophile has a different priority, then the configuration may also change. Compare the following two reactions:

The carbon on the right in the epoxide has the second priority because it is connected to an oxygen while the one in the cyclohexane ring is connected to two carbons and a hydrogen. This remains the same in the first reaction, however, in the second reaction, the carbon in the cyclohexane ring has a greater priority than the CH2-CN because of the two hydrogens.
So, always pay attention if the priorities of the groups are changing upon the addition of the nucleophile. What is perhaps more important is that the nucleophilic addition is always SN2 so the nucleophile is always on the opposite side of the epoxide oxygen. Wedge and dash and R and S may be misleading depending on how the molecule is drawn and how the priority of the groups is affected.
We have a separate post dedicated to the exceptions in SN1 and SN2 reactions which also address the common tricks with wedge and dash representation and absolute configurations, so feel free to check that out as well.
This is what we had to cover for the ring-opening reactions of epoxides under basic and acidic conditions. Check out this article on the summary of epoxide reactions as well as the practice problems to master the topic.
Check Also
- Preparation of Epoxides
- Ring-Opening Reactions of Epoxides
- Reactions of Epoxides Practice Problems
- The Grignard Reaction of Epoxides
- Naming Ethers
- The Williamson Ether Synthesis
- Reactions of Ethers-Ether Cleavage
