Reduction of Acyl Chlorides to Alcohols
Acid chlorides can be reduced to alcohols by lithium aluminum hydride (LiAlH4) and sodium borohydride (NaBH4).
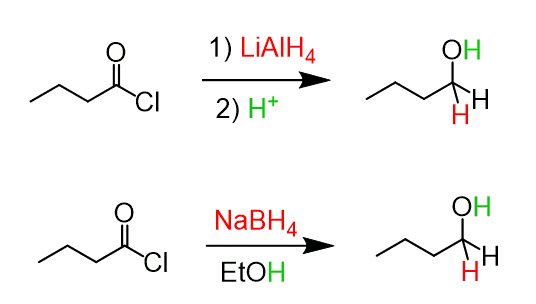
In both reactions, there is an aldehyde intermediate formed and therefore, the reducing agent is used in excess to shift the reaction to completion.
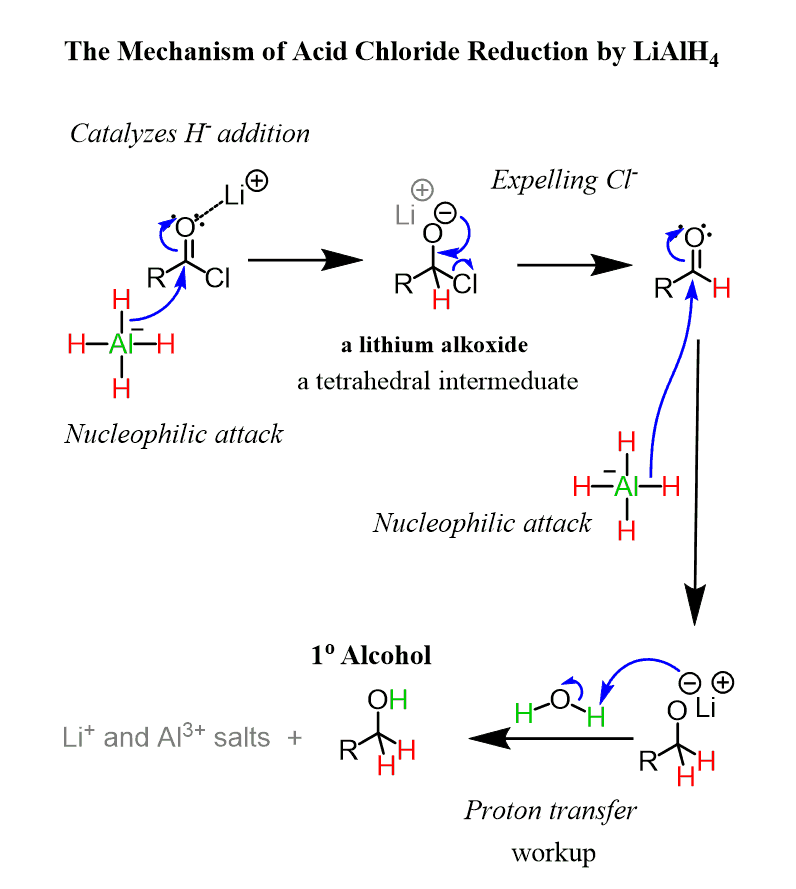
The mechanism of acid chloride reduction with LiAlH4 is similar to the one of esters:
Reduction of Acyl Chlorides to Aldehydes
We discussed earlier that it is not possible to stop the reduction at the aldehyde since this requires using only one equivalent of LiAlH4 and this will lead to a mixture of starting material, aldehyde and the alcohol.
Therefore, a less powerful reducing agent is needed to selectively convert acid chlorides to aldehydes. There are quite a few of those, with LiAl(OtBu)3H (Lithium tri(t-butoxy) aluminum hydride) being the most commonly seen in undergraduate courses:
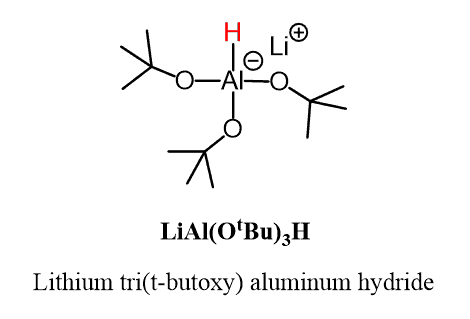
Lithium tri(t-butoxy) aluminum hydride LiAl(OtBu)3H is a derivative of LiAlH4 where three of the four hydrogens are replace with tBuO groups. This modification reduces the reactivity of the hydride ion and although it still rapidly reacts with the acid chloride, the slower reaction with the aldehyde allows to isolate from the reaction mixture:

The best yields are obtained when the reaction is carried out at -78oC (sublimation point of dry ice).
Check Also
- Preparation of Carboxylic Acids
- Naming Carboxylic Acids
- Naming Nitriles
- Naming Esters
- Naming Carboxylic Acid Derivatives – Practice Problems
- The Addition-Elimination Mechanism
- Fischer Esterification
- Ester Hydrolysis by Acid and Base-Catalyzed Hydrolysis
- What is Transesterification?
- Esters Reaction with Amines – The Aminolysis Mechanism
- Ester Reactions Summary and Practice Problems
- Preparation of Acyl (Acid) Chlorides (ROCl)
- Reactions of Acid Chlorides (ROCl) with Nucleophiles
- R2CuLi Organocuprates – Gilman Reagent
- Reaction of Acyl Chlorides with Grignard and Gilman (Organocuprate) Reagents
- Reduction of Carboxylic Acids and Their Derivatives
- Preparation and Reaction Mechanism of Carboxylic Anhydrides
- Amides – Structure and Reactivity
- Naming Amides
- Amides Hydrolysis: Acid and Base-Catalyzed Mechanism
- Amide Dehydration Mechanism by SOCl2, POCl3, and P2O5
- Amide Reduction Mechanism by LiAlH4
- Reduction of Amides to Amines and Aldehydes
- Amides Preparation and Reactions Summary
- Amides from Carboxylic Acids-DCC and EDC Coupling
- The Mechanism of Nitrile Hydrolysis To Carboxylic Acid
- Nitrile Reduction Mechanism with LiAlH4 and DIBAL to Amine or Aldehyde
- The Mechanism of Grignard and Organolithium Reactions with Nitriles
- The Reactions of Nitriles
- Converting Nitriles to Amides
- Carboxylic Acids to Ketones
- Esters to Ketones
- Carboxylic Acids and Their Derivatives Practice Problems
- Carboxylic Acids and Their Derivatives Quiz
- Reactions Map of Carboxylic Acid Derivatives
