Carboxylic acids can be reduced to primary alcohols using an excess of strong reducing agents such as lithium aluminum hydride (LiAlH4 or LAH).

As sodium borohydride (NaBH4) is the most common reducing agent we learn about together with LiAlH4, let’s emphasize that it is very inefficient in reducing carboxylic acids, so do not use it if you do not want to lose points on your exam.
Back to the LiAlH4 reduction: Aside from the acidic workup in the last step, the reduction is overall a three-step conversation. Remember that LIAlH4 is a strong base, so the first step is the deprotonation of the carboxylic acid. In the next two steps, we have a nucleophilic addition of the hydride to the carbonyl group, thus an excess of reducing agent is needed:

The formation of carboxylate ion after deprotonation is a good explanation for why NaBH4 is not effective in reducing carboxylic acids. Because of the resonance electron donation by the negatively charged oxygen, the carboxylate ion is not as electrophilic, and the addition of hydride requires a powerful reducing agent.
In the case of LiAlH4, the addition of hydride ion is facilitated by the coordination of the Al to the carbonyl oxygen. This withdraws some of the electron density thus making the carbon more electron-deficient. After the hydride addition, we have an elimination step where the –OAlH2 is expelled resorting the carbonyl in a form of an aldehyde:

Notice that aldehyde is another intermediate in this reaction, but we cannot stop the reduction at this stage. Remember, aldehydes are more reactive than carboxylic acids, esters, and especially carboxylate ions because there is no resonance donation of the oxygen to the carbonyl carbon which reduces the electron-deficient character of the carbonyl.

The greater reactivity of the aldehyde explains why t cannot be isolated as the final product.
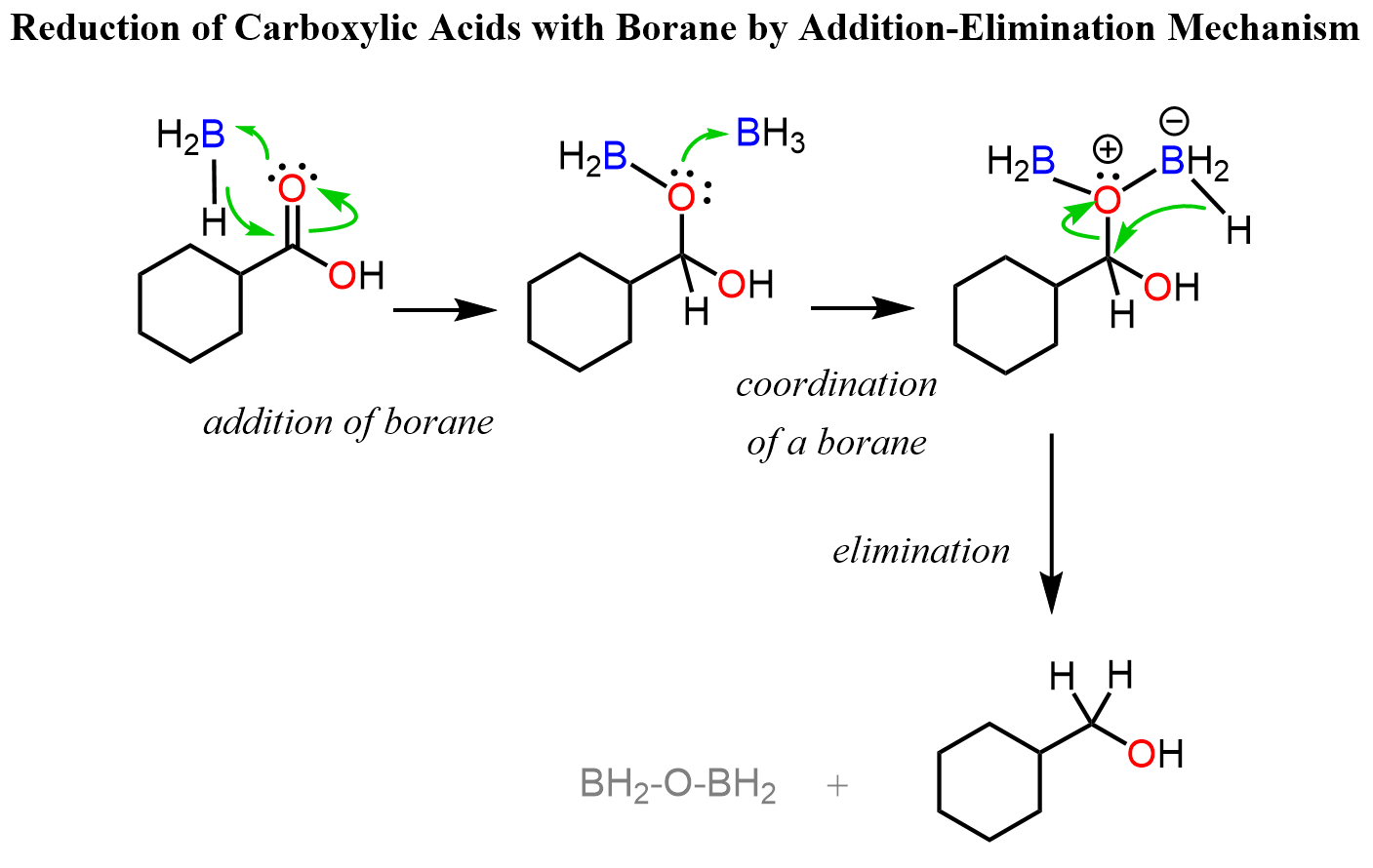
Reduction of Carboxylic Acids with Borane
We saw in the mechanism above that the carbonyl is activated by the coordination of the oxygen to Al which serves as a Lewis acid there. Now, borane is another Lewis acid, and when coordinated to an oxygen, it becomes electron-rich and thus a hydride donor. This turns out to be a good tool for reducing carboxylic acids to alcohol. It is still an addition-elimination reaction to the carbonyl, however, the intermediate is not an aldehyde by rather a boronic ester:
You can read more about it in different articles including https://doi.org/10.1016/j.jphotobiol.2015.04.015.
Check Also
- LiAlH4 and NaBH4 Carbonyl Reduction Mechanism
- Reduction of Carboxylic Acid Derivatives
- Alcohols from Carbonyl Reductions – Practice Problems
- Fischer Esterification
- Ester Hydrolysis by Acid and Base-Catalyzed Hydrolysis
- What is Transesterification?
- Esters Reaction with Amines – The Aminolysis Mechanism
- Ester Reactions Summary and Practice Problems
- Preparation of Acyl (Acid) Chlorides (ROCl)
- Reactions of Acid Chlorides (ROCl) with Nucleophiles
- Reaction of Acyl Chlorides with Grignard and Gilman (Organocuprate) Reagents
- Reduction of Acyl Chlorides by LiAlH4, NaBH4, and LiAl(OtBu)3H
- Preparation and Reaction Mechanism of Carboxylic Anhydrides
- Amides – Structure and Reactivity
- Naming Amides
- Amides Hydrolysis: Acid and Base-Catalyzed Mechanism
- Amide Dehydration Mechanism by SOCl2, POCl3, and P2O5
- Amide Reduction Mechanism by LiAlH4
- Amides Preparation and Reactions Summary
- Amides from Carboxylic Acids-DCC and EDC Coupling
- The Mechanism of Nitrile Hydrolysis To Carboxylic Acid
- Nitrile Reduction Mechanism with LiAlH4 and DIBAL to Amine or Aldehyde
- The Mechanism of Grignard and Organolithium Reactions with Nitriles
- Carboxylic Acids to Ketones
- Esters to Ketones
- Carboxylic Acids and Their Derivatives Practice Problems
- Carboxylic Acids and Their Derivatives Quiz
