In the previous post, we saw that amides can be reduced to amines by LiAlH4:
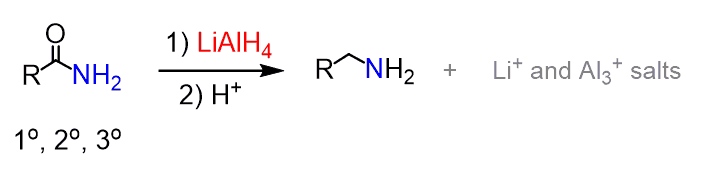
Let’s see how tertiary amides can be reduced to amines and aldehydes using alkyboranes.
Reduction of Amides by Alkylboranes
The reduction of amides by alkyl boranes such as 9-BBN or Sia2BH is not common in undergraduate textbooks, however, I thought it is worth mentioning here since we can selectively convert the amide to an amine or an aldehyde.
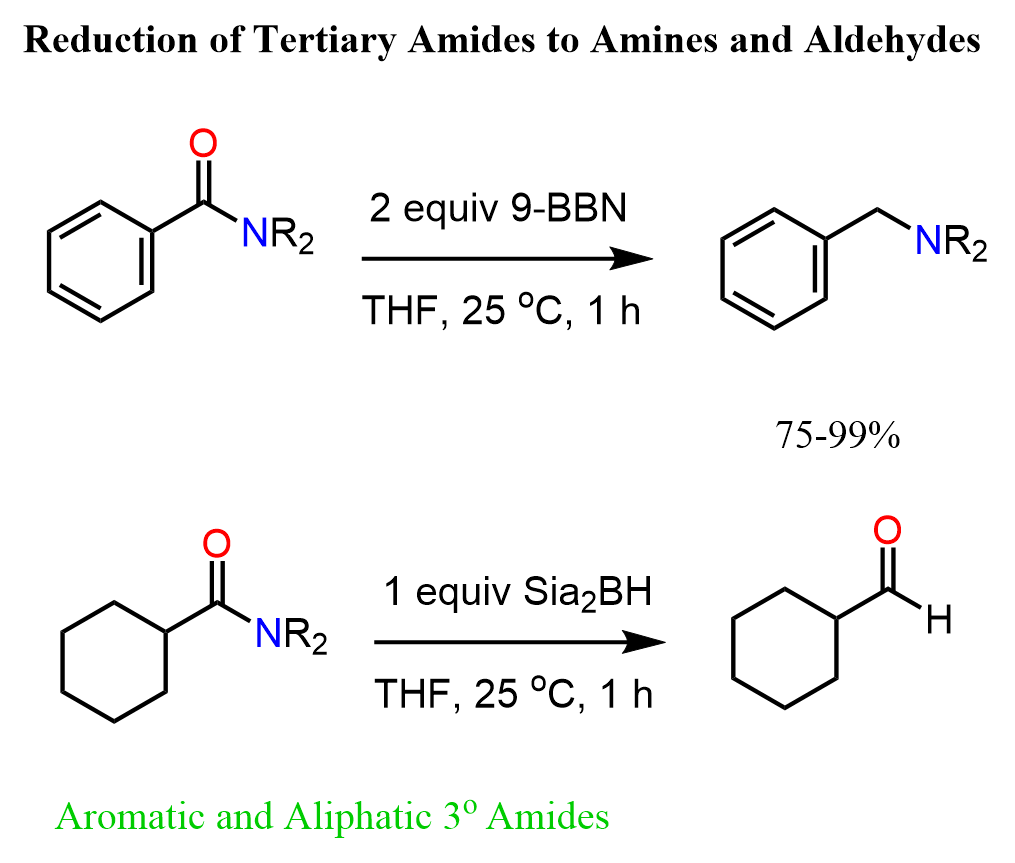
It is mostly related to tertiary amides but I have not done a thorough research on whether primary or secondary amides can be used as well. In general, one problem with primary and secondary amides is the formation of O-B and N-B esters with boron (J. Am. Chem. Soc. 1970, 92, 24, 7161–7167). There are studies on reducing secondary amides using NIckel catalyst so, feel free to check this article and the references for insight into the topic.
9-BBN: Tertiary Amides to Amines
9-BBN and Sia2BH are bulky alkyl boranes which we have seen earlier in the hydroboration-oxydation of alkynes.
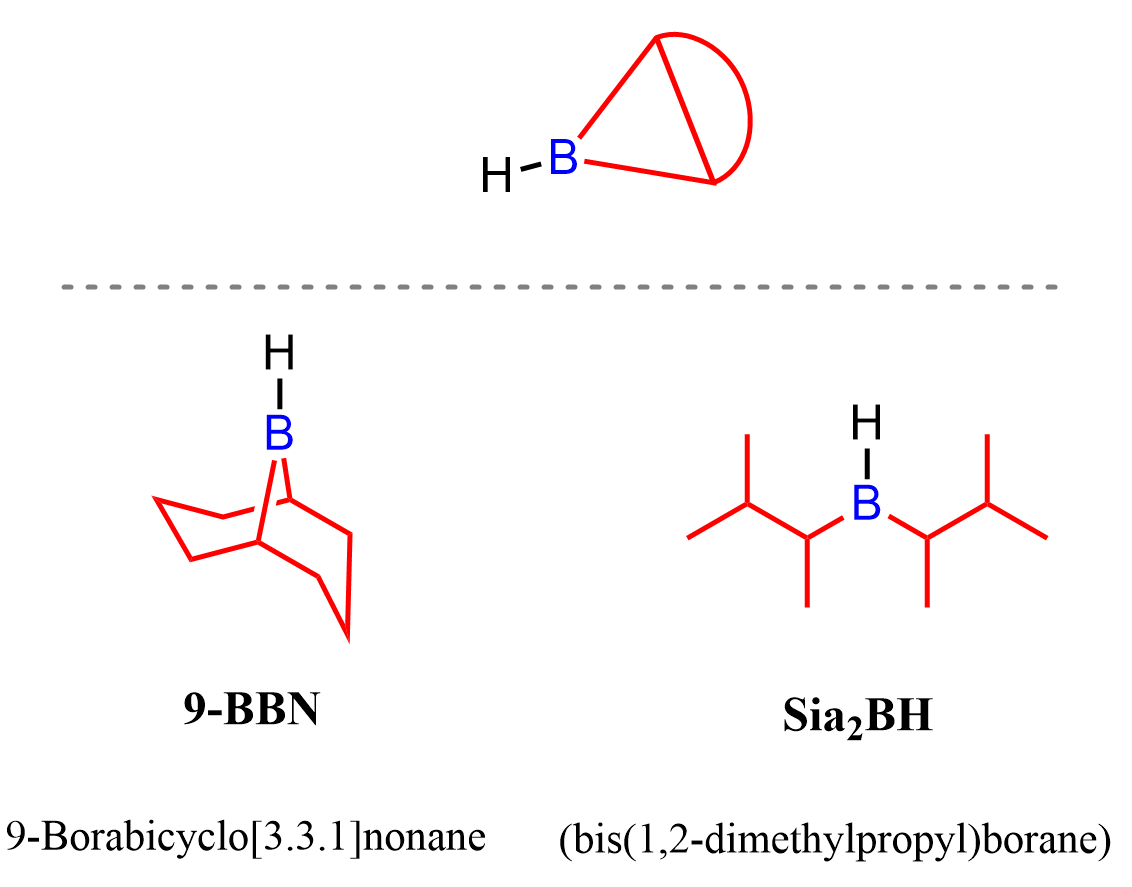
The idea behind using alkyl borane or borohydrides in the reactions of alkenes, alkynes, and carbonyl compounds is the fact that boron has an empty p orbital which makes it electrophilic. The electron source can be any pi bond, and when the electrons are added to the boron, it now becomes electron-rich which makes it a donor of a hydride ion. We have seen this in many reduction reactions with NaBH4, and also with BH3 (B2H6) for the reduction of carboxylic acids.
Taking into consideration all the patterns we have seen in the reduction reaction, let’s try to put together a plausible mechanism for the reduction of a tertiary amide to an amine:
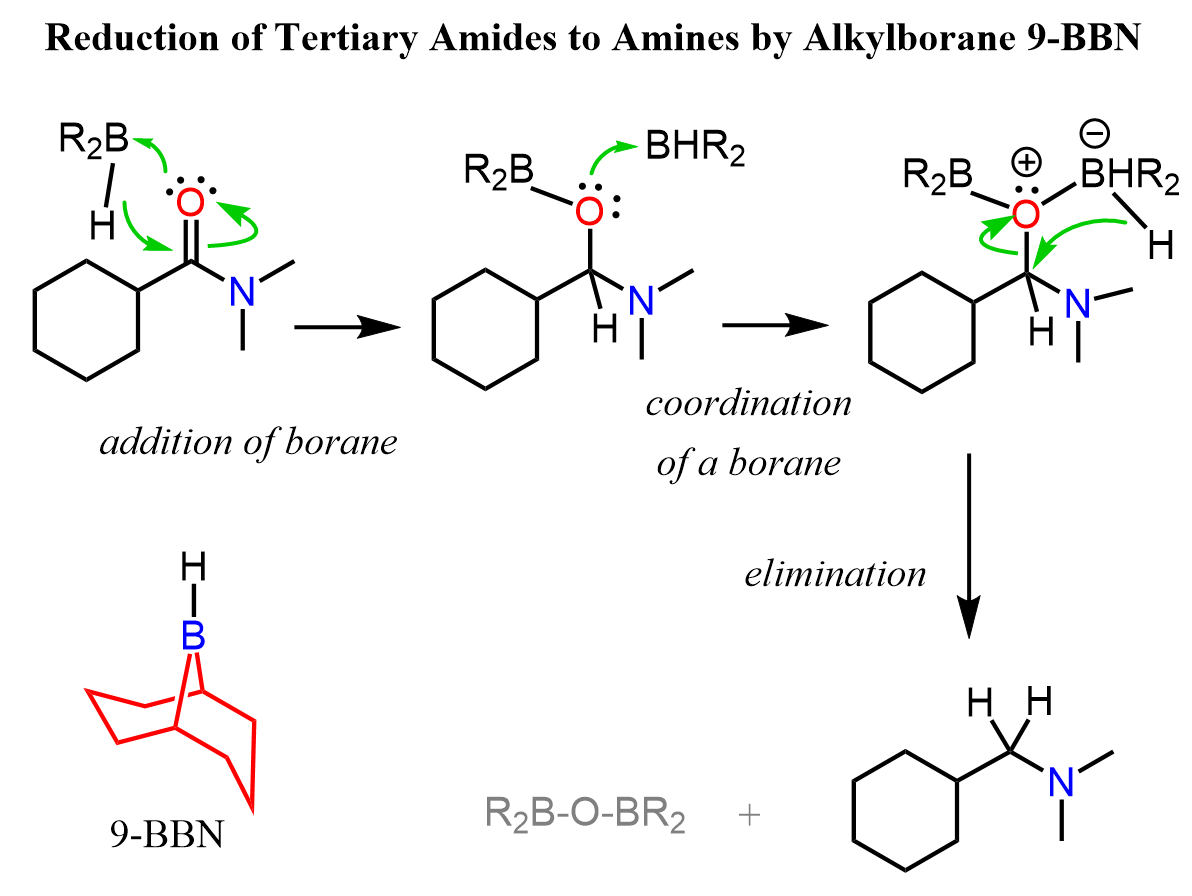
In the first step, we have an addition to the carbonyl via coordination of the boron forming a boronic ester intermediate. After another coordination, the boronic ester is converted into a good leaving group by the positively charged oxygen and it is kicked out by the second hydride addition.
Sia2BH: Tertiary Amides to Aldehydes
Although structurally very similar to 9-BBN, it has been shown that the reduction of tertiary amides can be stopped at aldehydes when Sia2BH is used. This means the second hydride addition does not occur and the hydrolysis of the boronic ester intermediate gives the corresponding aldehyde as the final product:
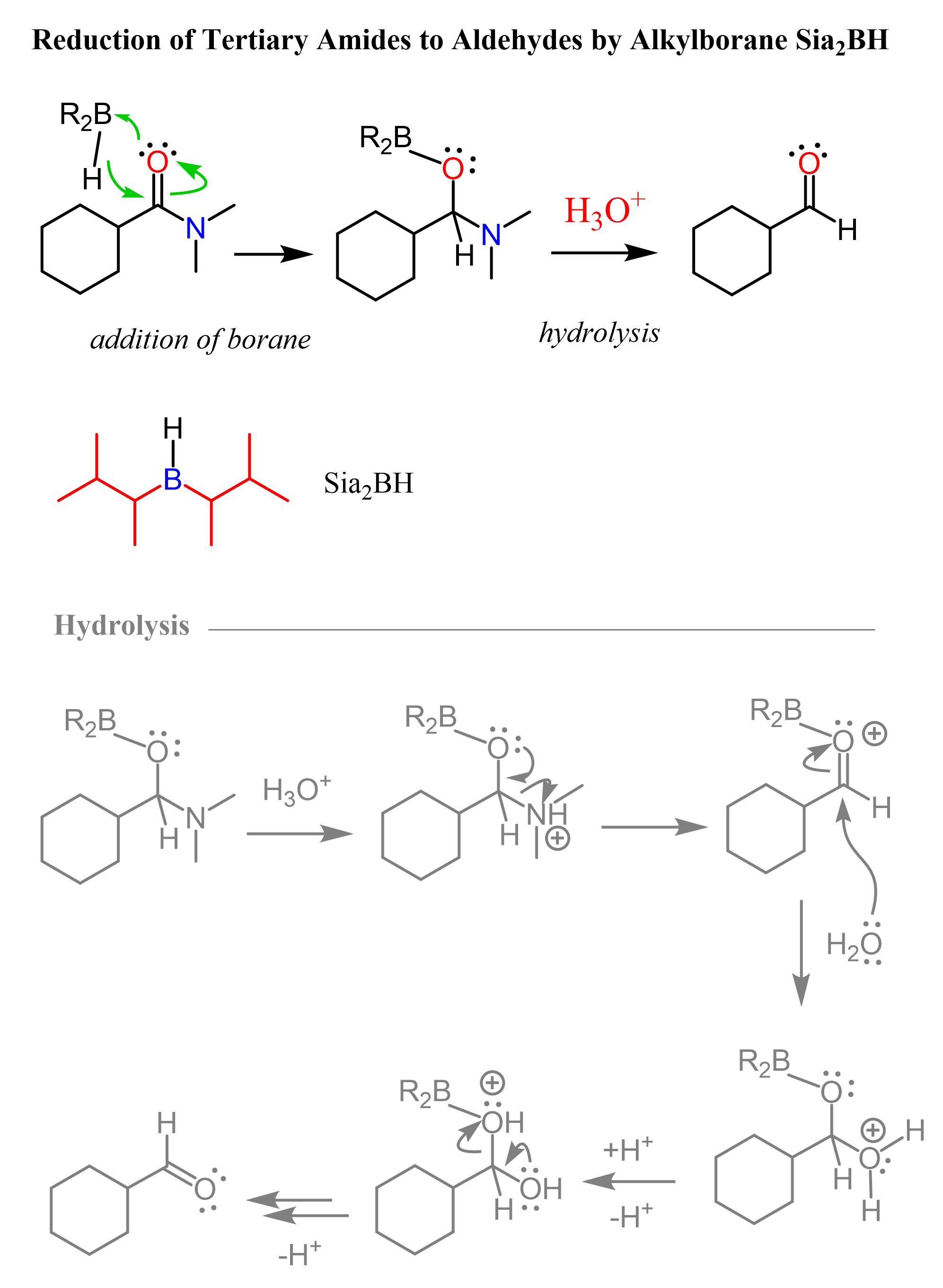
Here is the original article for further reading: Tetrahedron Letters, Vol. 38, No. 10, pp. 1717-1720, 1997.
Need some good practice on the reactions of carboxylic acids and their derivatives?
Check this 45-question, Multiple-Choice Quiz with a 50-minute Video Solution covering the reactions of acids, esters, lactones, amides, acid chlorides etc.
Carboxylic Acids and Their Derivatives Quiz
Check Also
- Carboxylic Acids and Their Derivatives Practice Problems
- Preparation of Carboxylic Acids
- Fischer Esterification
- Ester Hydrolysis by Acid and Base-Catalyzed Hydrolysis
- What is Transesterification?
- Esters Reaction with Amines – The Aminolysis Mechanism
- Ester Reactions Summary and Practice Problems
- Preparation of Acyl (Acid) Chlorides (ROCl)
- Reactions of Acid Chlorides (ROCl) with Nucleophiles
- Reaction of Acyl Chlorides with Grignard and Gilman (Organocuprate) Reagents
- Reduction of Acyl Chlorides by LiAlH4, NaBH4, and LiAl(OtBu)3H
- Preparation and Reaction Mechanism of Carboxylic Anhydrides
- Amides – Structure and Reactivity
- Amides Hydrolysis: Acid and Base-Catalyzed Mechanism
- Amide Dehydration Mechanism by SOCl2, POCl3, and P2O5
- Amide Reduction Mechanism by LiAlH4
- Amides Preparation and Reactions Summary
- The Mechanism of Nitrile Hydrolysis To Carboxylic Acid
- Nitrile Reduction Mechanism with LiAlH4 and DIBAL to Amine or Aldehyde
- The Mechanism of Grignard and Organolithium Reactions with Nitriles

