Reductive amination is used for preparing amines from aldehydes and ketones. All we need is to mix an amine with an aldehyde or ketone in the presence of sodium cyanoborohydride (NaBH3CN) at lower pH levels:

So, how does this work? Remember, we have seen earlier that the reaction of primary amines with aldehydes and ketones produces imines, and secondary amines form enamines:

One great feature of the C=N bond is that it can be reduced using hydrogen in the presence of metal catalysts or NaBH3CN. The advantage of using NaBH3CN is that it allows for a one pot synthesis without harsh conditions such as elevated pressure and temperature:

The Mechanism of Reductive Amination
The mechanism of reductive amination is not much different from what we have seen in the reduction of carbonyl compounds using NaBH4 and NaBH3CN. The carbon of the C=N bond has a partial positive charge because of the greater electronegativity of nitrogen, and this makes it an electrophilic center susceptible to the nucleophilic attack by the hydride ion:

The last step, the reduction of iminium ion, is what we also see in the reduction of nitriles with LiAlH4.
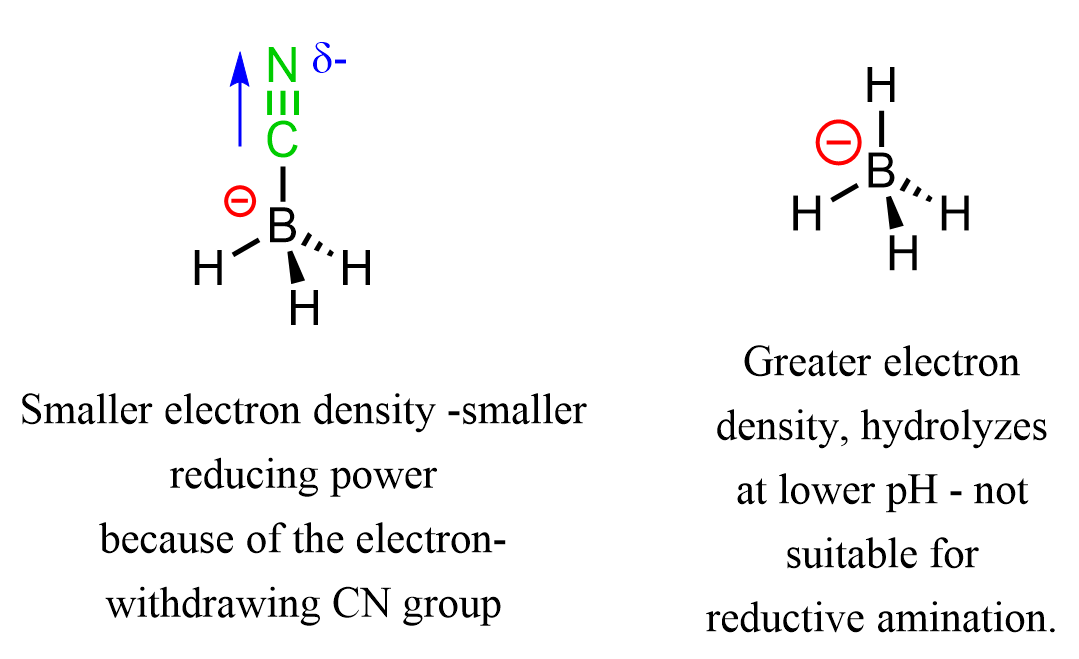
One question you may be wondering is why we cannot use LiAlH4 or NaBH4. The problem with LiAlH4 is that it requires dry conditions, and NaBH4 hydrolyzes at lower pH as well. So how is NaBH3CN different? The role of the CN group in NaBH3CN is the reduction of the electron density and thus the reducing power through inductive and resonance withdrawing effects:
Patterns in Reductive Amination
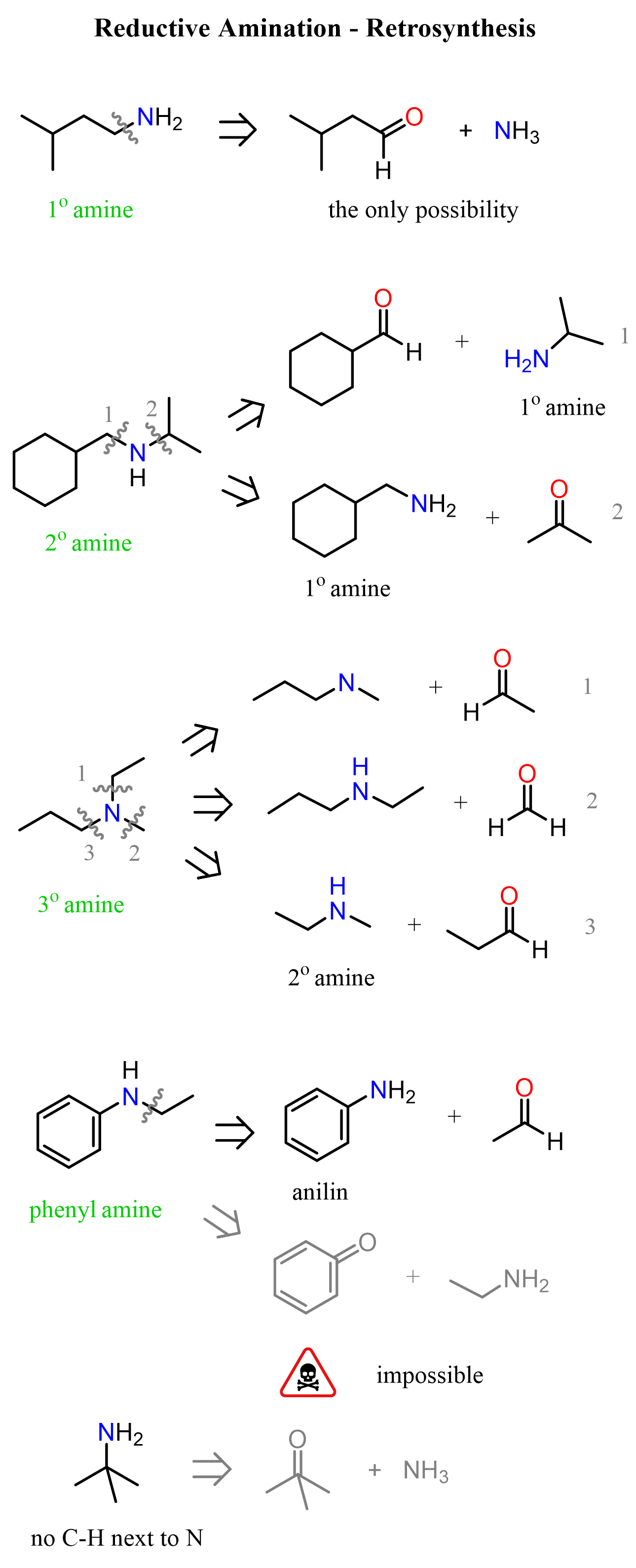
The main requirement for an amine to be used in reductive amination is the presence of a hydrogen on the nitrogen. This restricts the pool of amines to ammonia, primary, and secondary amines. Remember, primary amines give imines, and secondary amines give enamines, whereas tertiary amines do not undergo condensation with carbonyl compounds, and thus cannot be used in reductive amination.

One again, notice that when a primary amine is used, the intermediate that undergoes reduction by NaBH3CN is the imine, whereas in the case of secondary amines, it is the iminium ion salt.
The only amine that would lead to a primary amine through reductive amination is ammonia. Most often, ammonium chloride or acetate is used as a source of ammonia. So, for any secondary and tertiary amine, we can retrosynthetically identify two pairs of amines and carbonyls as precursors:
These were the main points I wanted to include in this post on the reductive amination. Let me know if you have questions or if there is anything you find that would be worth adding here.
Check Also
- Nomenclature of Aldehydes and Ketones
- How to Name a Compound with Multiple Functional Groups
- Preparation of Aldehydes and Ketones
- Nucleophilic Addition to Carbonyl Groups
- Reactions of Aldehydes and Ketones with Water
- Reactions of Aldehydes and Ketones with Alcohols: Acetals and Hemiacetals
- Acetals as Protecting Groups for Aldehydes and Ketones
- Formation and Reactions of Imines and Enamines
- Acetal Hydrolysis Mechanism
- Imine and Enamine Hydrolysis Mechanism
- Hydrolysis of Acetals, Imines, and Enamines-Practice Problems
- Reaction of Aldehydes and Ketones with CN, Cyanohydrin Formation
- The Wittig Reaction: Examples and Mechanism
- The Wittig Reaction: Practice Problems
- Aldehydes and Ketones to Carboxylic Acids
- Reactions of Aldehydes and Ketones – Practice Problems
- Aldehydes and Ketones Reactions Practice Quiz
- Reactions Map of Aldehydes
- Reactions Map of Ketones
