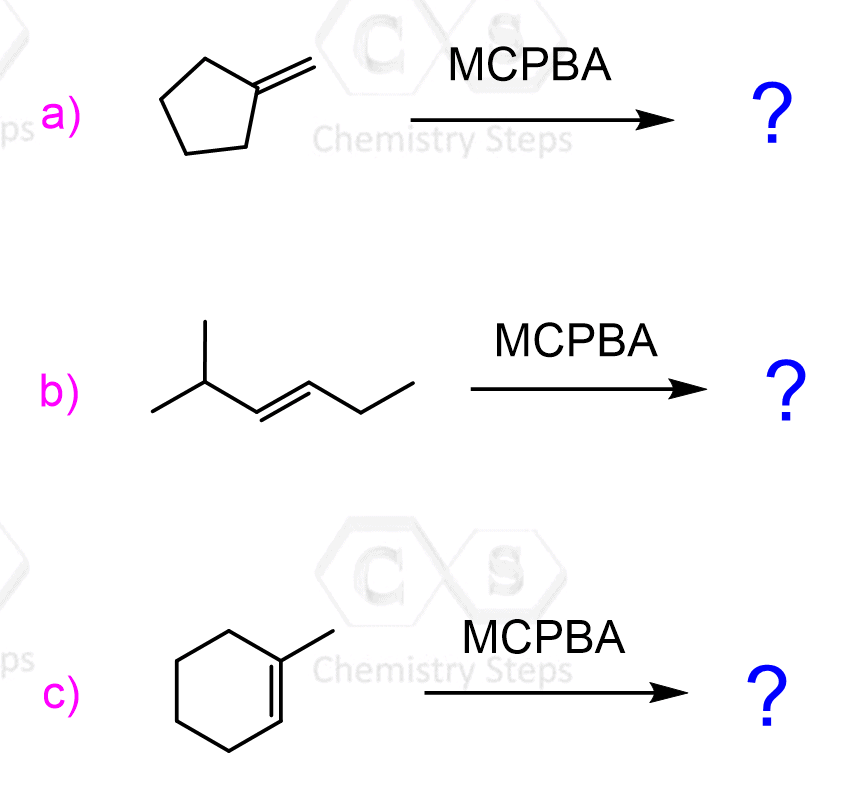
Epoxides are three-membered rings containing an oxygen atom. They are associated with high ring strain, and this is the basis of their reactivity towards nucleophiles despite lacking a good leaving group.
The ring strain is a combination of two destabilizing factors: angle strain and torsional strain. The angle strain is a result of poor orbital overlap caused by a deviation from the ideal bond angle of 109.5o characteristic for sp3-hybridized tetrahedral atoms.
In addition to the angle strain, three-membered rings have high torsional strain since the hydrogens are almost permanently eclipsed due to the locked sigma bonds between the carbons:

These features make peroxides undergo ring-opening reactions with strong or weak nucleophiles.

There are some differences when an epoxide reacts with a strong nucleophile (basic conditions) and a weak nucleophile (acidic conditions), and we will go over them in the next sections.
Epoxide Ring-Opening with Strong Nucleophiles
The ring-opening reactions of epoxides occur via SN2 mechanism where the oxygen of the epoxide is the leaving group. The product contains an alkoxy group and therefore, an aqueous or mild acidic work-up is necessary to obtain neutral species:

Once again, even though alkoxide is not a good leaving group, as we know from the nucleophilic substitution reactions, the reaction occurs because of the driving force which is the highly strained epoxide ring.
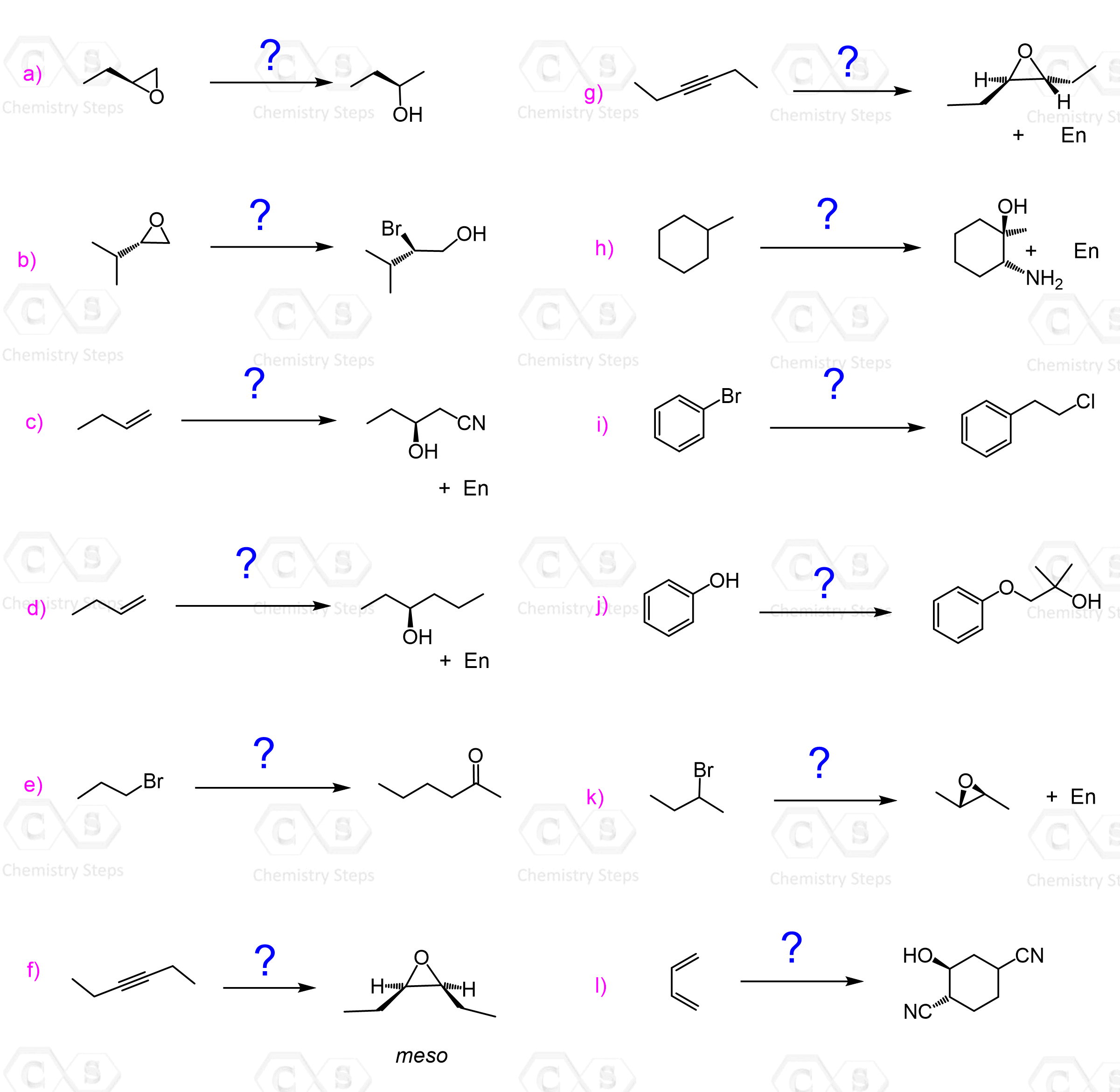
There are many nucleophiles that will react with epoxides in this fashion. Some common examples are hydroxides, thiols, cyanides, Grignard reagents, and LiAlH4. We will go over the details and mechanism of these reactions, but first, check below a Summary of Ring-Opening Reactions of Epoxides with Strong and Weak Nucleophiles:

Let’s start with the two types of mechanisms for ring-opening reactions.
The Mechanism of Epoxide Ring-Opening Reactions
The arrows we drew above for the ring-opening with strong nucleophiles suggest an SN2 mechanism as the nucleophilic attack and the loss of a leaving group (ring-opening) happen at the same time.

On the other hand, when a weak nucleophile is used in acidic conditions, the nucleophilic attack occurs at the more substituted carbon which is consistent with the SN1 mechanism. However, there is no carbocation formed before the nucleophilic attack, and therefore, the mechanism is thought to be somewhere in-between SN1 and SN2 (see below).
Epoxide Ring-Opening with Weak Nucleophiles
These are mainly the HX acids, which first protonate the epoxide, thus making it more reactive since the oxygen is now a better leaving group. After the protonation, the halide attacks and opens the epoxide ring, forming an alcohol with an adjacent halide:
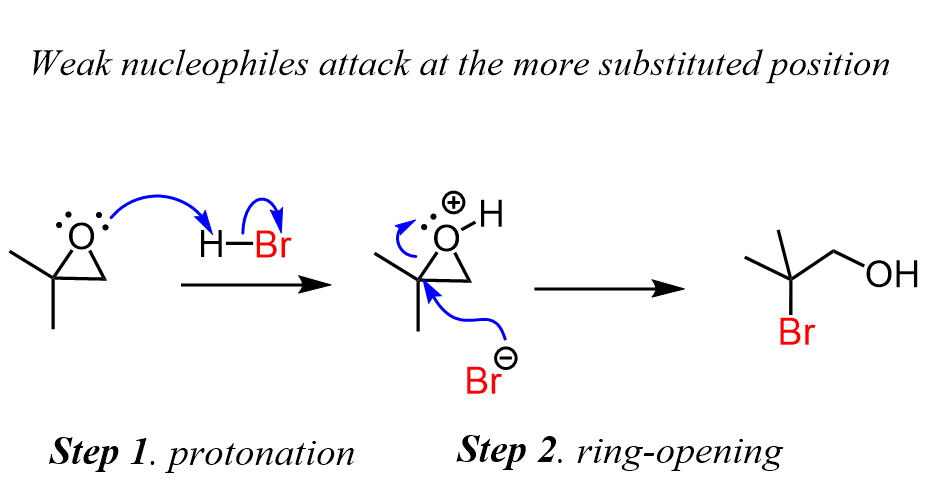
Notice that the order of steps is reversed compared to what we have seen with strong nucleophiles.
And, even though the reaction seems to follow an SN2 mechanism when an unsymmetrical epoxide is used, the regiochemical outcome suggests a possible SN1 path as well.
- Unsymmetrical epoxides react with weak nucleophiles at the more substituted carbon atom.
With this said, let’s dive into the regiochemistry, the stereochemistry, and the mechanistic aspects of the epoxide ring-opening reactions.
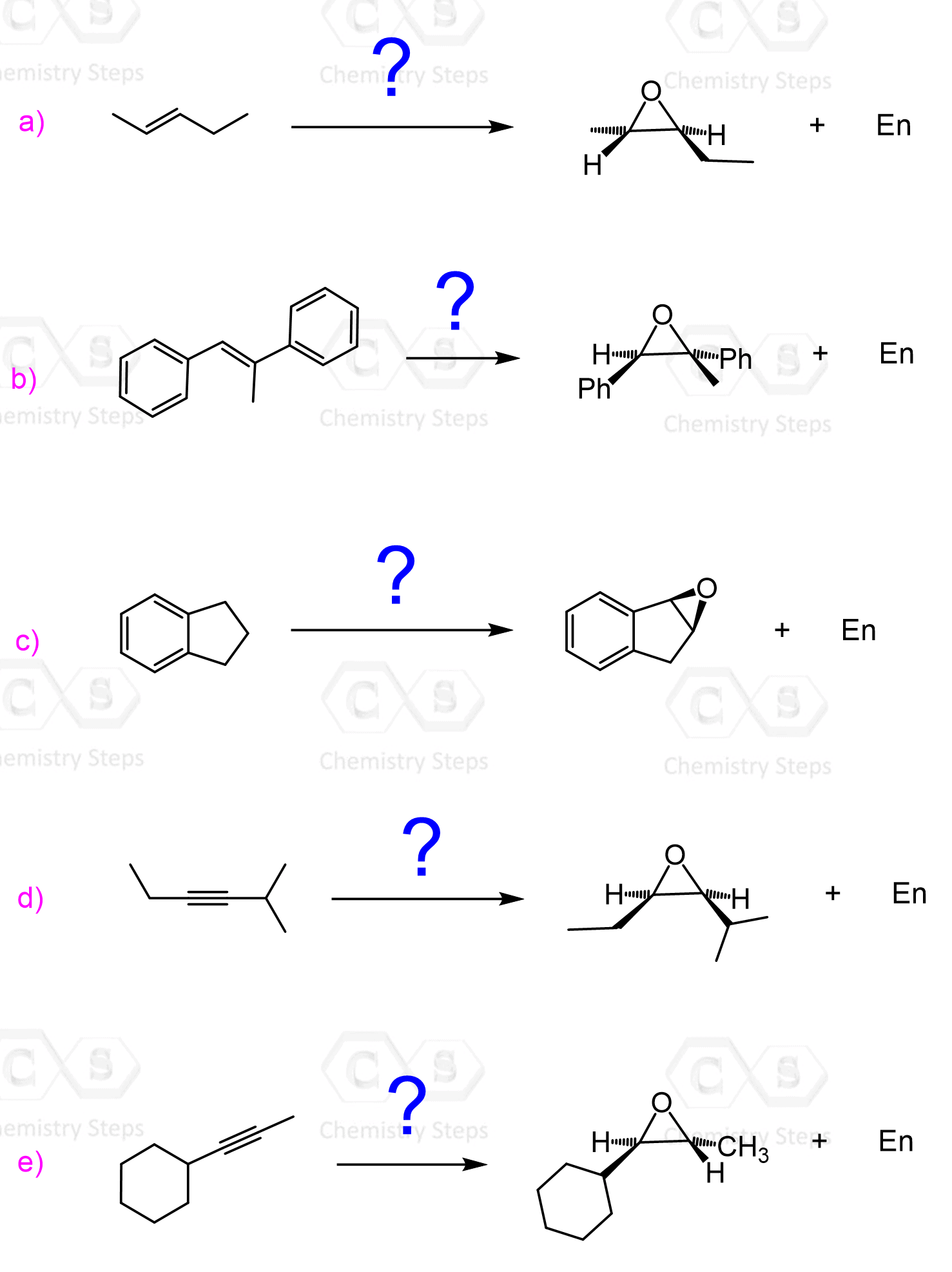
The Regiochemistry of Epoxide Reactions with Strong Nucleophiles
When unsymmetrical epoxides are reacted, strong nucleophiles attack at the less substituted (less hindered) position.

This is explained simply by the fact that the less substituted carbon is more accessible by the nucleophile and therefore, reacts faster, just like we learned in the SN2 mechanism.
The regiochemistry of Epoxide Reactions with Weak Nucleophiles
Unlike the reaction with strong nucleophiles, unsymmetrical epoxides react at the more substituted carbon atom when a weak nucleophile is used. The regioselectivity is best observed when a tertiary carbon atom is present in the epoxide:

We can explain this by the stability of the two possible transition states. The first transition state has a partial positive charge on a more substituted carbon, making it more stable.

This is the electronic effect (electron and charge distribution), whereas the effect on reactivity based on the degree of substitution is the steric effect, which is what we used to explain the regiochemistry when strong nucleophiles are used. However, it works along with the electronic effect, and how the reaction proceeds depends on which of the factors predominates.
For example, epoxides with a secondary and primary carbon atom connected to the oxygen give a mixture of regioisomers:

These epoxides, with primary and secondary positions, favor the reaction at the less substituted carbon, meaning that the steric effect dominates here. This indicates that the transition state in this case does not resemble a carbocation as much as it does when a tertiary carbon is present. This is expected because of the higher stability of tertiary carbocations.
To summarize, the regiochemistry of epoxide ring-opening reactions is regioselective for either a strong nucleophile or an acid.
This selectivity of unsymmetrical epoxides, however, is exactly the opposite as.
- Strong nucleophiles react at the less substituted position, while
- Weak nucleophiles react at the more substituted position (3o carbons)
The Stereochemistry of Epoxide Reactions
Both strong and weak nucleophiles open the epoxide ring by an opposite-side nucleophilic attack. This puts the nucleophile and the alkoxy group on opposite sides and as a result, trans or anti-products are always formed.
If an achiral epoxide is the starting material, either an achiral product or a racemic mixture of enantiomers is formed. For example, the reaction between a Grignard reagent and an achiral epoxide produces an achiral alcohol:

Let’s also consider a reaction on achiral epoxide producing a racemic mixture of enantiomers. As an example, let’s consider the ring opening of meso-1,2-epoxycyclohexane. Remember, meso compounds are achiral, but they do have chiral centers, and when the ring is opened, one of the C-O bonds is broken, and an unsymmetrical product is formed. Therefore, although the mixture is achiral, it consists of two enantiomers:

Notice that the carbon where the attack occurs changes the configuration, whereas the other one stays as it was.
If both carbons of the epoxide connected to the oxygen are chiral, then the product is also going to be chiral. Inversion of configuration occurs at the carbon where the nucleophile attacks. For example, if one enantiomer of 1,2-Epoxy-1-methylcyclohexane is used, then the nucleophile can more easily attack the less substituted carbon, which leads to the formation of one of the enantiomers (major product):

This is all we had to cover for the reactions of epoxides, and as usual, a handful of examples as practice problems can be found here!
Check Also
- Preparation of Epoxides
- Reactions of Epoxides under Acidic and Basic Conditions
- Reactions of Epoxides Practice Problems
- The Grignard Reaction of Epoxides
- Naming Ethers
- The Williamson Ether Synthesis
- Reactions of Ethers-Ether Cleavage