In the previous post, we talked about the Michael reaction which is a conjugate-addition reaction of doubly stabilized enolates such as malonic ester, acetoacetic ester and the like with ɑ, β-unsaturated carbonyl compounds:
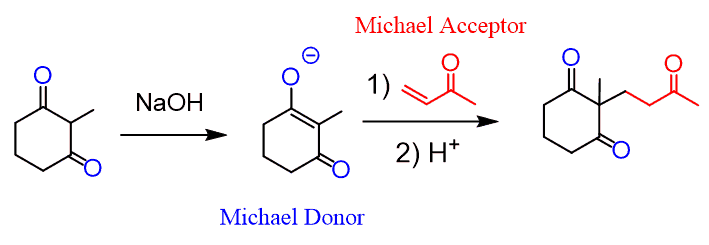
One important variation of the Michael addition is the Robinson annulation, which is commonly used in organic synthesis to prepare a ring through an aldol condensation of the Michael addition product.

So, what happens is the Michael product undergoes an intramolecular aldol condensation to form a new six-membered ring. Let’s put together a complete mechanism for the Robinson annulation:

The last step, after the aldol condensation, is an E1CB elimination, and the mechanism for this reaction is covered in the aldol condensation post.
The reaction works mostly for five- and six-membered rings as these are the most stable.
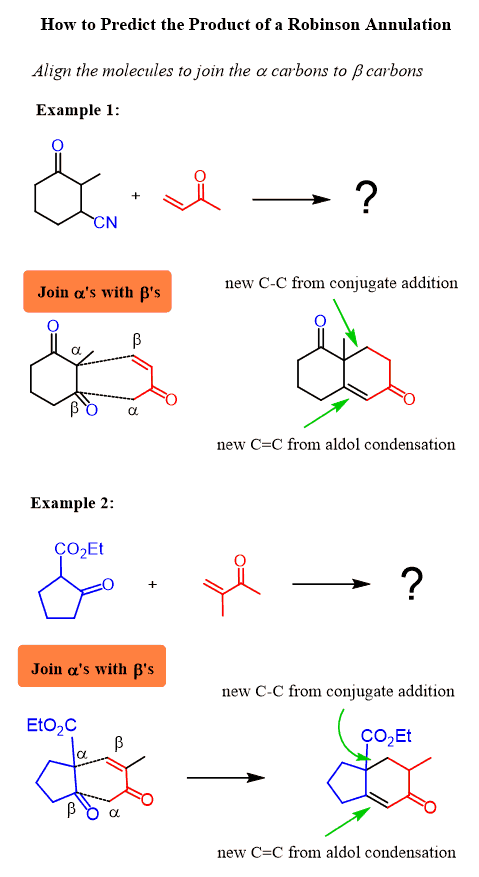
How to Predict the Product of a Robinson Annulation
One shortcut to predicting the product of a Robinson annulation reaction is to put the β carbon of the Michael acceptor next to the ɑ position of the Michael donor and the ɑ carbon of the Michael acceptor next to the carbonyl of the Michael donor. After this connect the carbons with a single and double bond respectively.
This may sound too much for a shortcut, but really, all you do is place ɑ and β carbons of the two molecules next to each other to make new bonds. I call it the α-β orientation:
Retrosynthetic Analysis in Robinson Annulation
To determine the starting materials of a Robinson annulation, you need to cleave the C=C bond and the bond between the β carbon and the carbon to which it is bonded. Luckily these bonds are arranged such that the bond cleavage is easily achieved by drawing a line that bisects both bonds:

Need some additional practice problems? Check this comprehensive set of alpha carbon chemistry questions:
Enolates in Organic Synthesis – a Comprehensive Practice Problem
Check Also
- Alpha Halogenation of Enols and Enolates
- The Haloform and Iodoform Reactions
- Alpha Halogenation of Carboxylic Acids
- Alpha Halogenation of Enols and Enolates Practice Problems
- Aldol Reaction – Principles and Mechanism
- Aldol Condensation – Dehydration of Aldol Addition Product
- Intramolecular Aldol Reactions
- Aldol Addition and Condensation Reactions – Practice Problems
- Crossed Aldol And Directed Aldol Reactions
- Crossed Aldol Condensation Practice Problems
- Alkylation of Enolates Alpha Position
- Enolate Alkylation Practice Problems
- Acetoacetic Ester Synthesis
- Acetoacetic Ester Enolates Practice Problems
- Malonic Ester Synthesis
- Michael Reaction: The Conjugate Addition of Enolates
- Robinson Annulation, Shortcut, and Retrosynthesis
- Claisen Condensation
- Dieckmann condensation – An Intramolecular Claisen Reaction
- Crossed Claisen and Claisen Variation Reactions
- Claisen Condensation Practice Problems
- Stork Enamine Synthesis
- Enolates in Organic Synthesis – a Comprehensive Practice Problem
