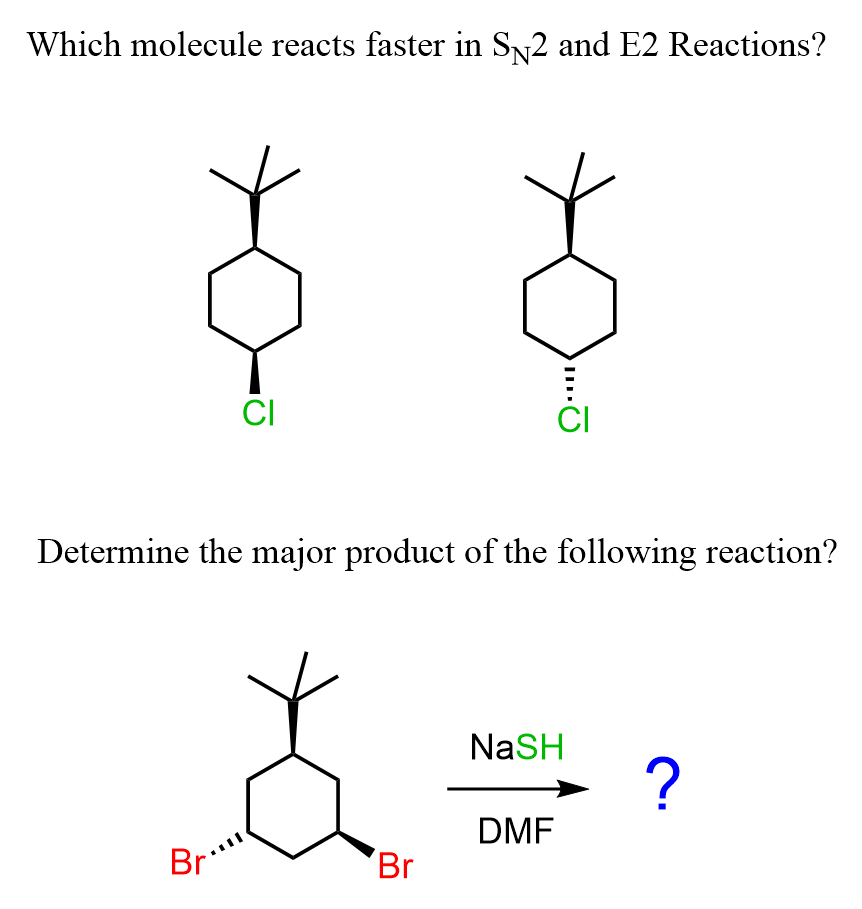
Let’s address two common exam questions: comparing the SN2 and E2 rates of two isomeric cyclohexanes and determining the major product in those reactions.
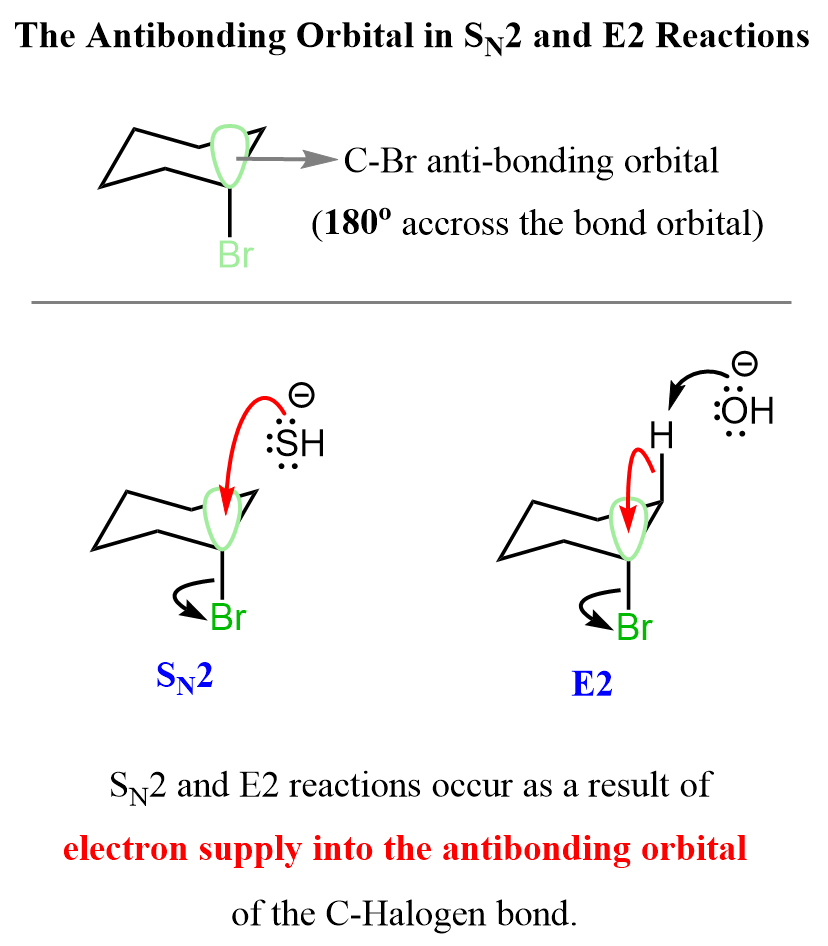
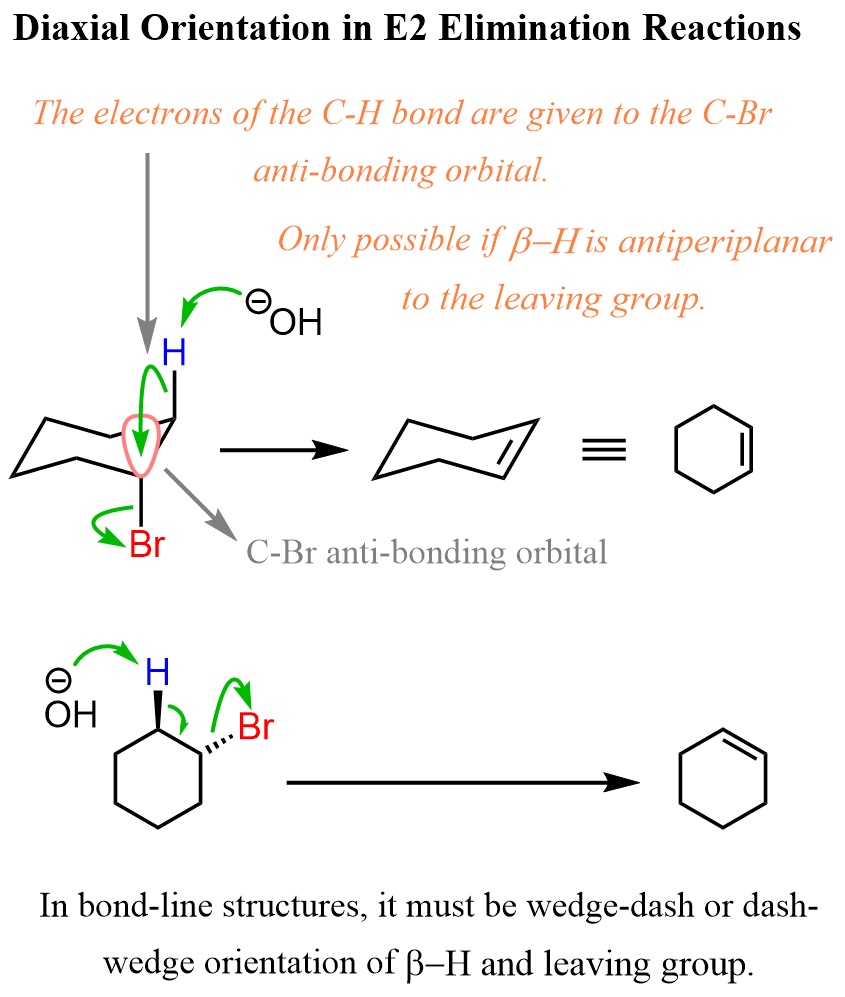
The first thing you need to remember here is that both SN2 and E2 reactions occur as a result of electrons moving into the antibonding orbital of the C-Halogen bond. It can also be any other leaving group such as for example a mesylate or a tosylate. The antibonding orbital of the C-X bond is 180o across the C-X bonding orbital. This is also known as the lowest unoccupied molecular orbital (LUMO).
In the case of the SN2 substitution, the source of electrons supplied into the antibonding orbital is from the nucleophile, whereas in E2 reactions, it is the electrons in the C-H (β hydrogen) bonding orbital.
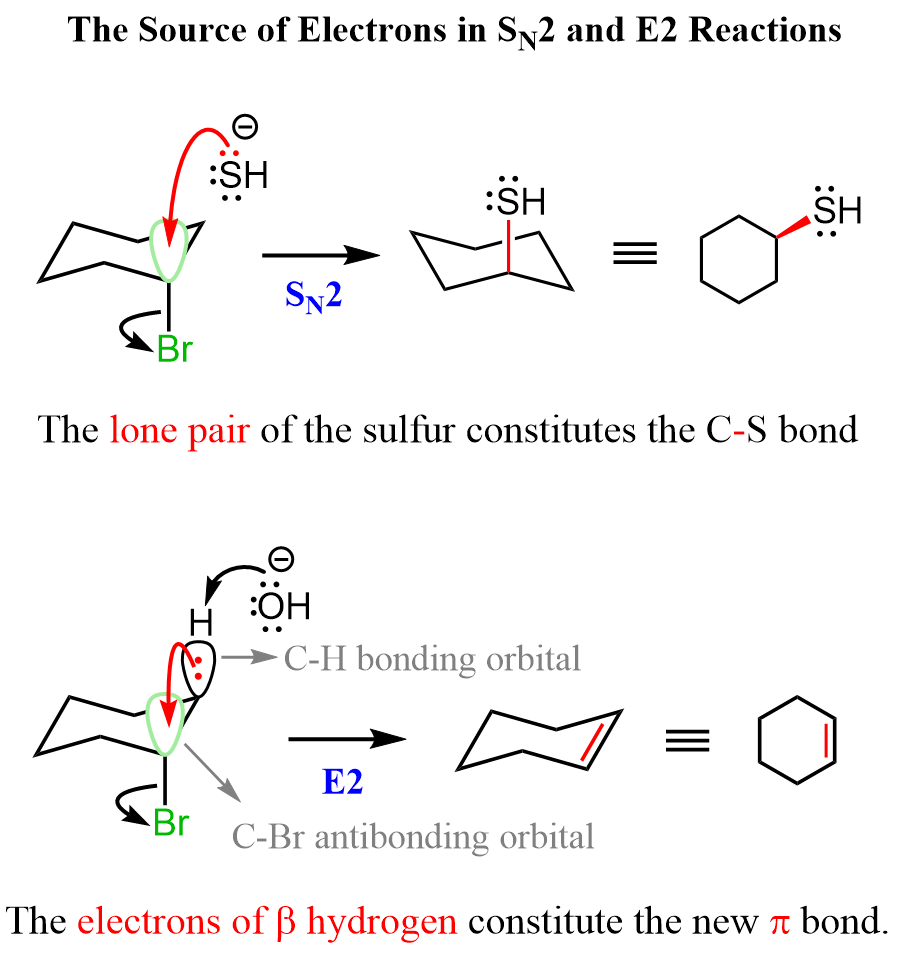
As the SN2 reaction proceeds forward, the electrons of the nucleophile become its new sigma with the carbon. In the case of E2 elimination, the electrons of the C-H bond become the newly-forming C=C π bond.
The Antiperiplanar Orientation
Let’s now mention the important geometrical requirement for this electron transfer to the antibonding orbitals. For SN2 substitution, the nucleophile must approach 180o from the leaving group (backside attack), and for the E2 reaction, the β hydrogen must be at 180o to the leaving group. This orientation is known as anti-periplanar orientation (recall the anti conformation from Newman projections). This is true for any cyclic or acyclic substrate:
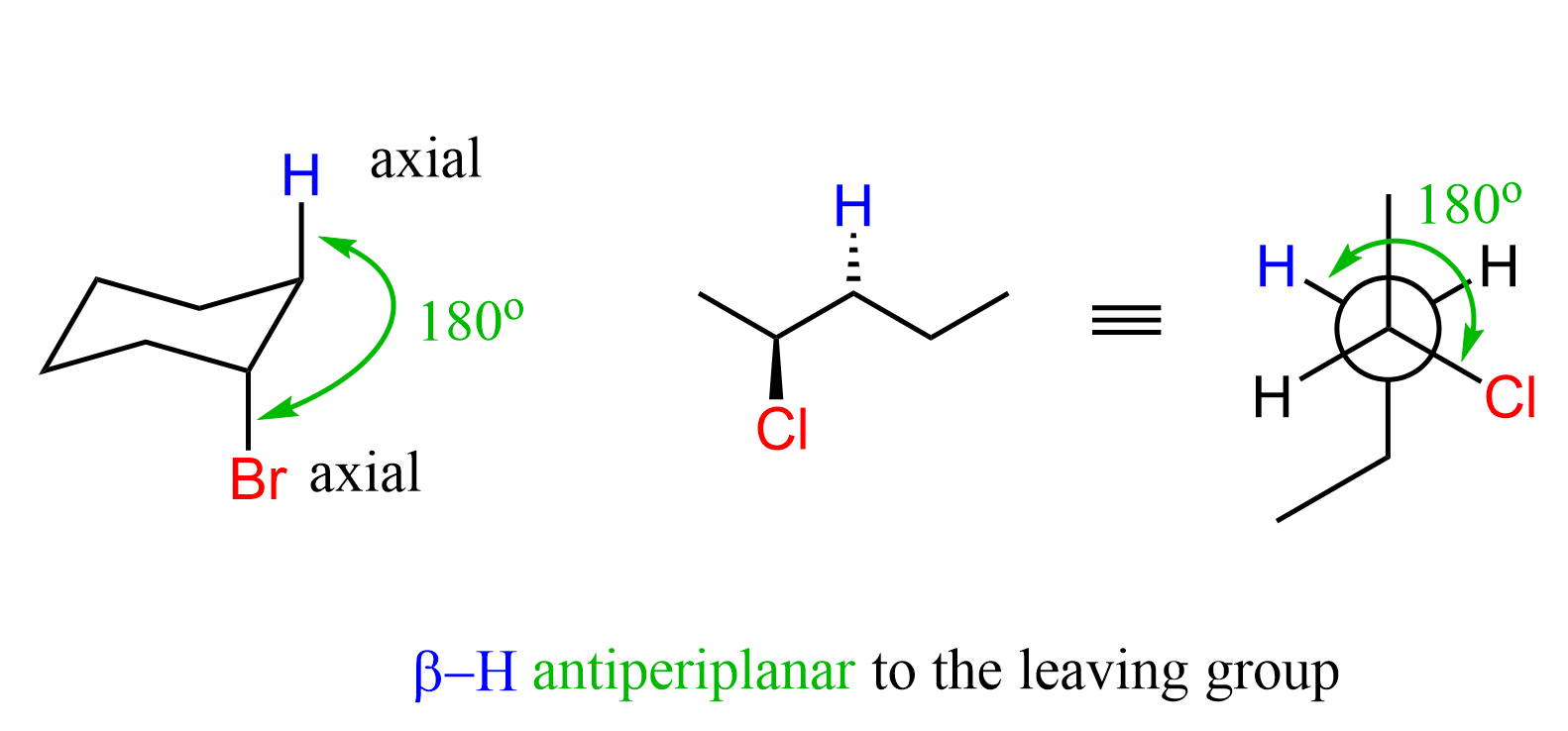
The geometry of sp3 carbons in chair conformations is such that Notice that the leaving group and the β hydrogen are antiperiplanar only when they are both in axial positions. So, the axial orientation of the leaving group and the β hydrogen is a must for the E2 elimination to occur.
The axial position of the leaving group is not a requirement as such in the case of SN2 reactions; however, it is still the preferred orientation of the leaving group, and we will see the reason for this in the next section.
Axial Position of the Leaving Group in SN2 Substitution
The nucleophile can access the antibonding orbital of the C-X bond both when it is in the axial or equatorial position. The word “axial” is perceived with negative association as we learn that it is unstable, unfavorable and etc., and because of this, one may assume that the chair conformation where the leaving group is equatorial must react faster in SN2 substitutions.
However, it turns out that the antibonding orbital of the C-X bond is more accessible to the nucleophile if it is in an axial position.
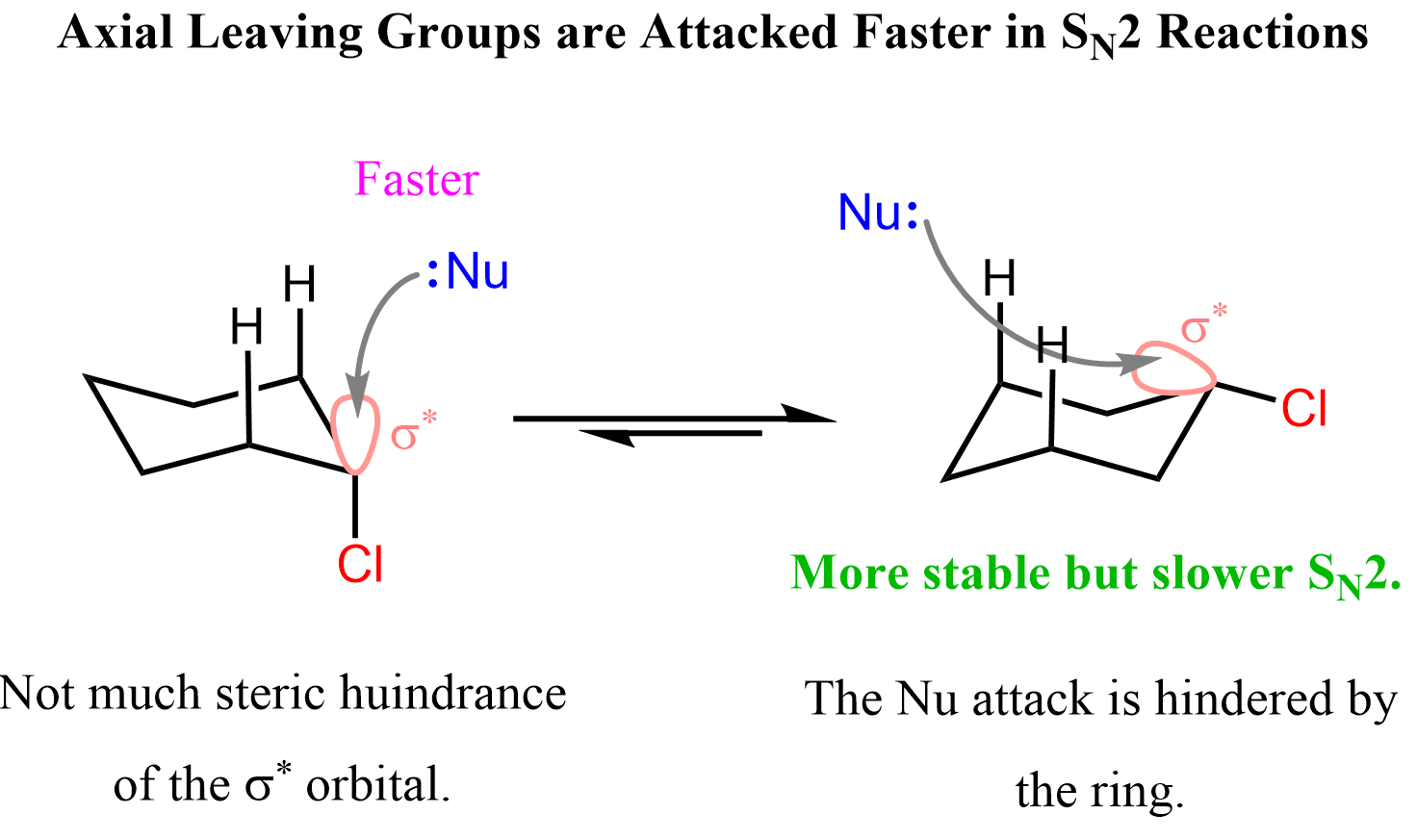
Notice in the picture below that when in the axial position, there is not much hindrance to the antibonding orbital, whereas when it is in the equatorial position, the nucleophile must slide over the entire ring to access it at the correct angle. Therefore, in general, the conformation where the leaving group is axial reacts faster in SN2 reactions.
The epoxidation of trans-2-bromocyclohexan-1-ol in Williamson Ether Synthesis is a notable example demonstrating the diaxial (anti-periplanar) orientation of the nucleophilic and the leaving group. The axial position of the bromine allows for a good alignment of HOMO and LUMO orbitals for the substitution to occur:

Although the equilibrium is favored for the conformation with the two groups being equatorial, the diaxial conformation quickly undergoes a substitution reaction, thus moving the process forward.
Which Cyclohexane will Undergo SN2 and E2 Reaction Faster?
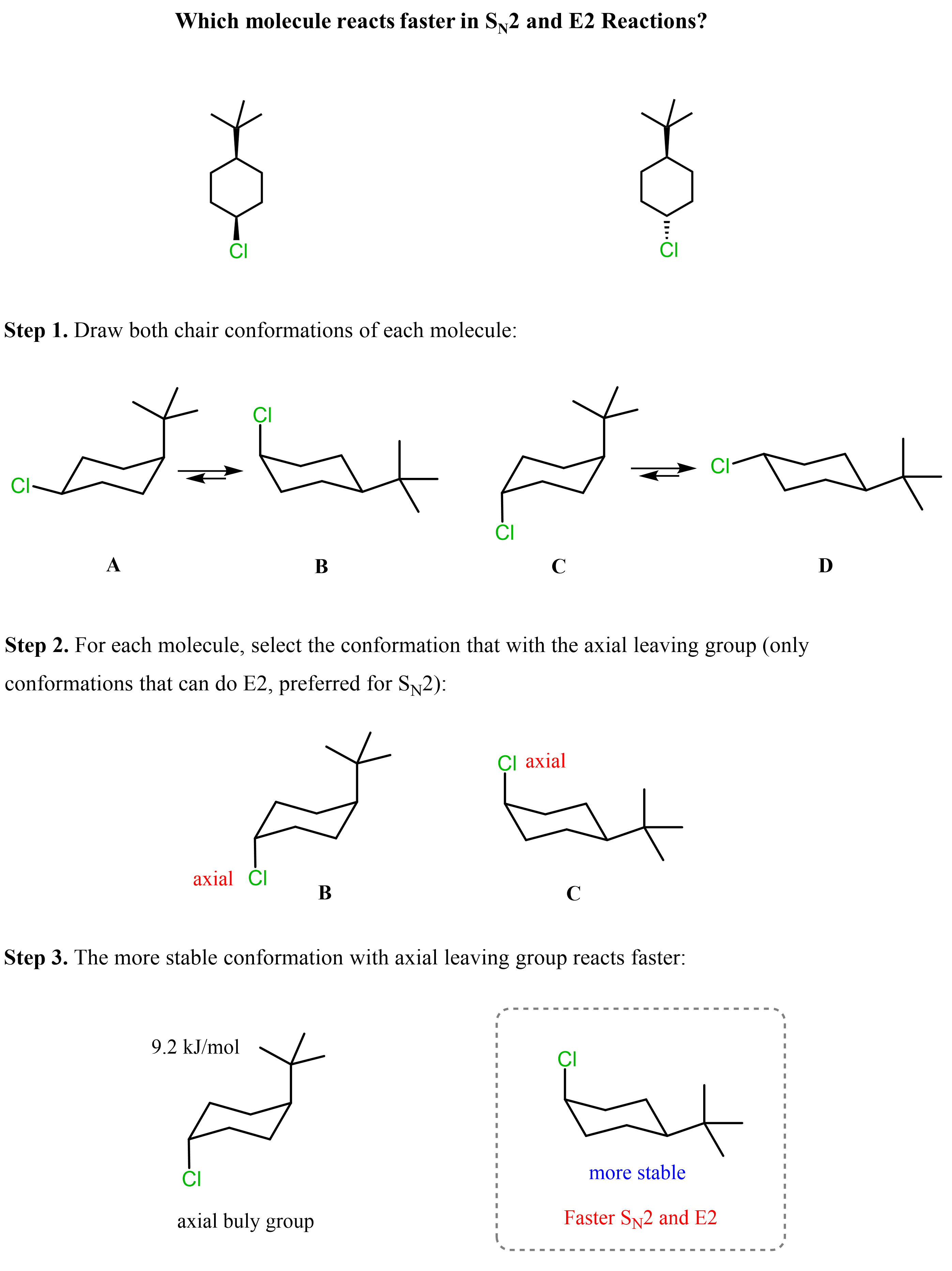
So far, our discussion has been about the orientation of the nucleophile, the base, the leaving group, and the geometry of the chair conformation. Let’s now compare two molecules, and, using this knowledge base, determine which isomer of the following cyclohexane undergoes faster SN2 substitution.
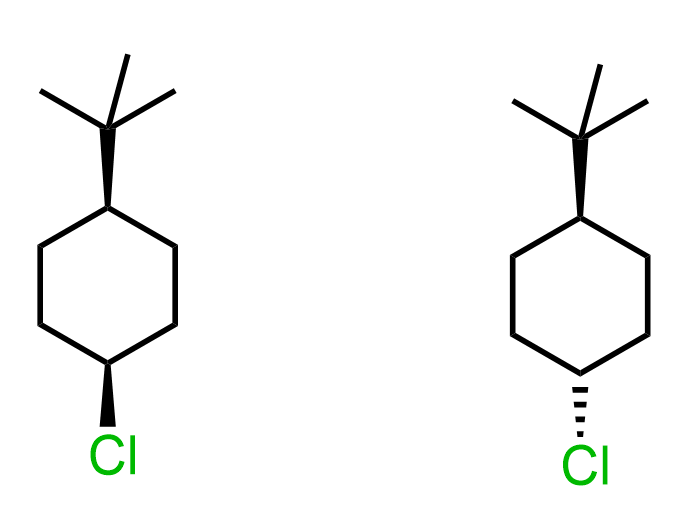
To do this, for each molecule, we need to draw the conformation where the Cl is axial and compare its relative stabilities. The one that is more stable will be present in higher concentration, thus reacting faster in SN2 and E2 reactions.
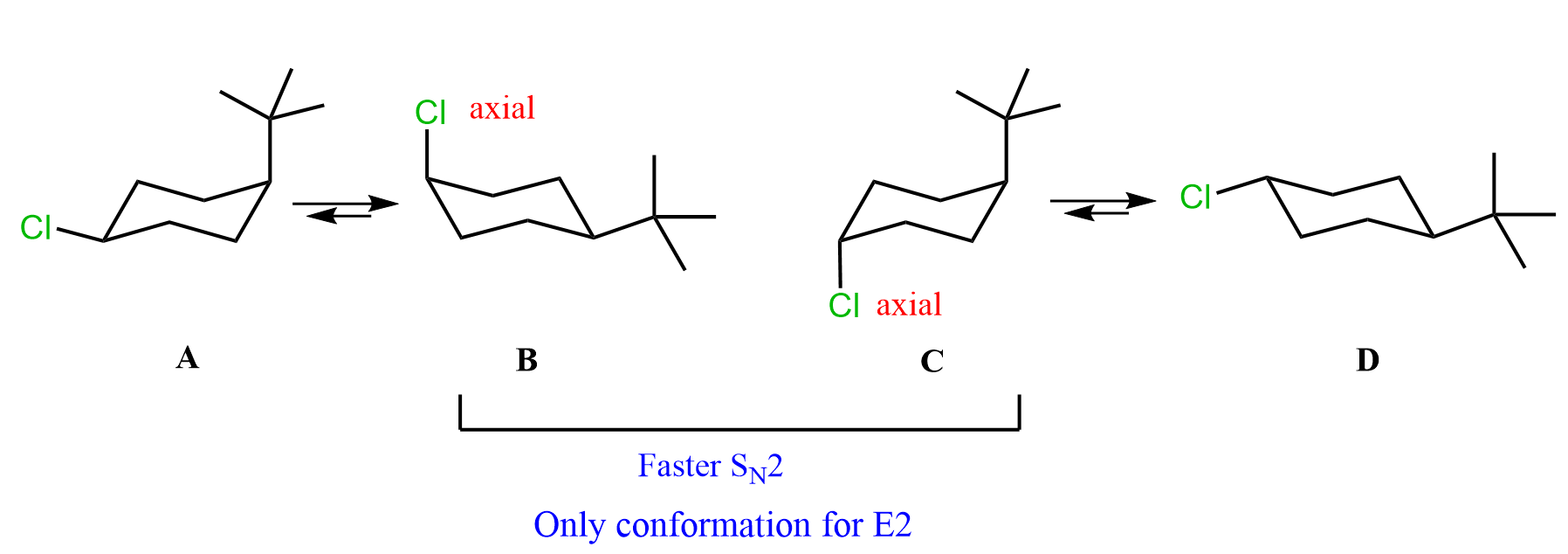
B and C are the two conformations with axial Cl, so we are comparing which of these two will react faster in SN2 and E2 reactions.
Next, we need to compare the orientation of the tert-butyl group. It is a bulky group, and because of 1,3-diaxial interactions, it is quite unstable (+9.2 kJ/mol of destabilization) when in the axial position. Therefore, conformation C is less stable than conformation B, which means conformation B of the first molecule will react faster in SN2 and E2 reactions.
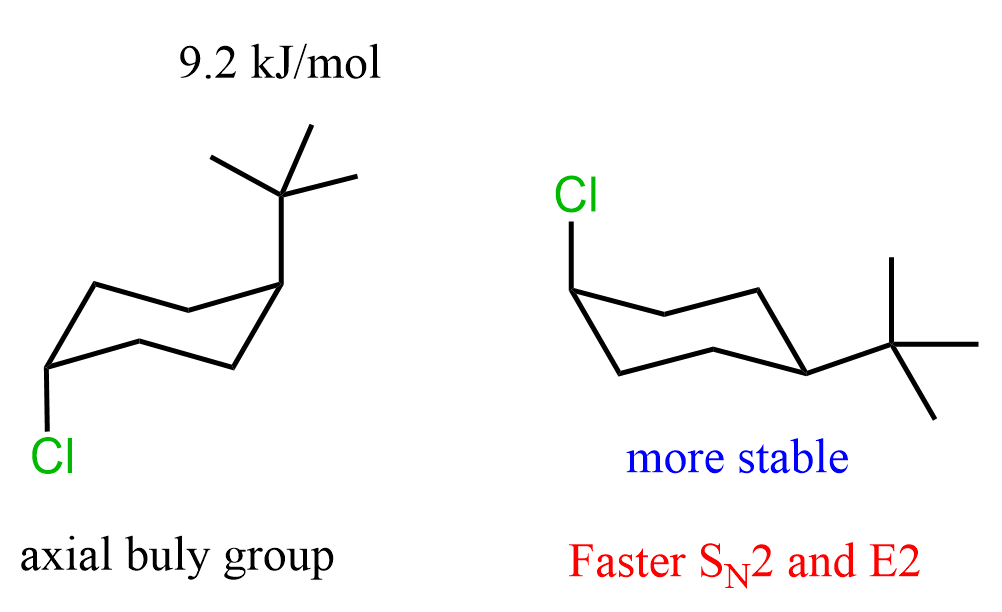
To summarize what we have discussed for determining the cyclohexane that undergoes faster SN2 and E2 reaction, draw both chair conformations of each molecule, then compare the stability of the conformations where the leaving group is axial: the more stable conformation with the axial leaving group reacts faster:
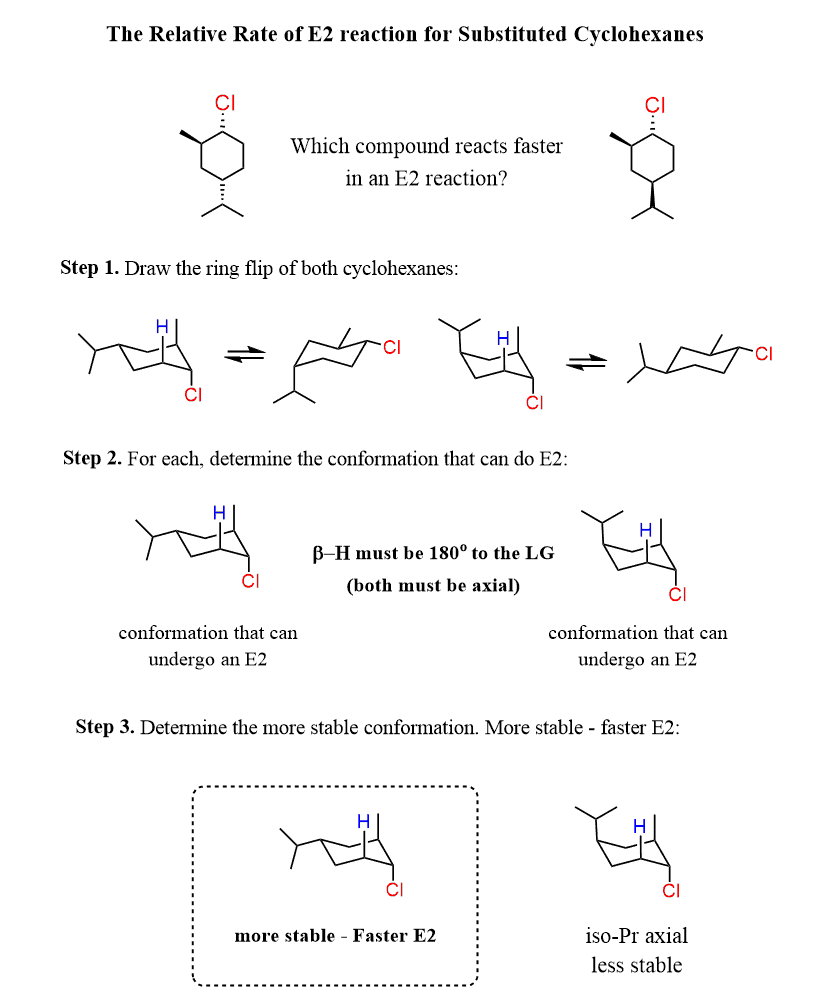
Let’s also discuss an example of cyclohexenes with two alkyl groups.
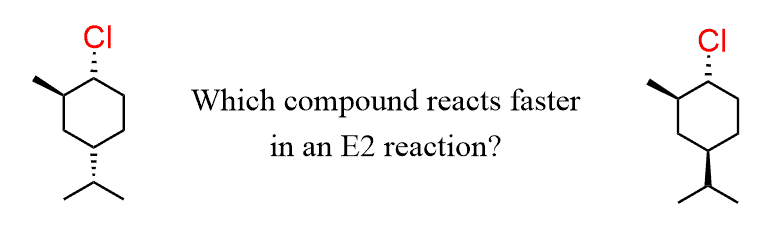
The rate of the reaction depends on the stability of the chair conformation with a trans diaxial arrangement of the leaving group and the corresponding β-hydrogen.
When the chlorine is in the axial position, the isopropyl group of the first molecule is in the equatorial position, and it is the more stable conformation; therefore, it will react faster in E2 elimination.
The Major Product of Cyclohexane SN2 Reactions
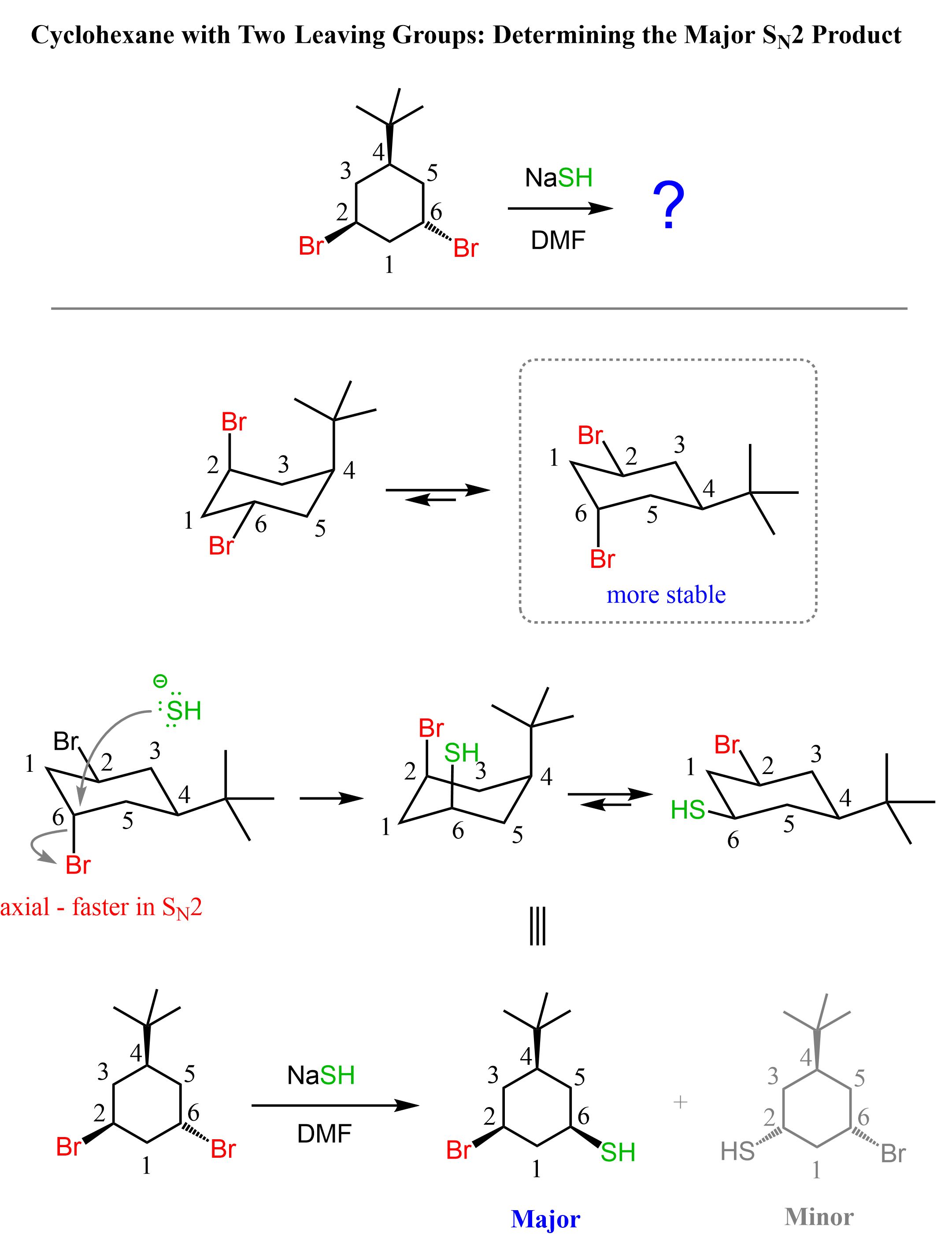
Let’s determine the major product of the SN2 reaction when the following cyclohexane is treated with a good non-basic nucleophile, NaSH:
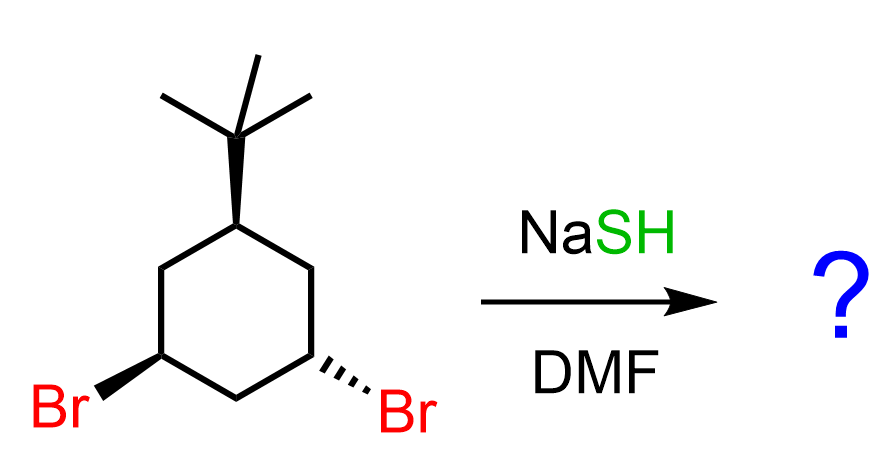
As always, we are going to determine the more stable chair conformation of the cyclohexane where the leaving group is in an axial position. Let’s number the ring so we can specify which bromine we are talking about:
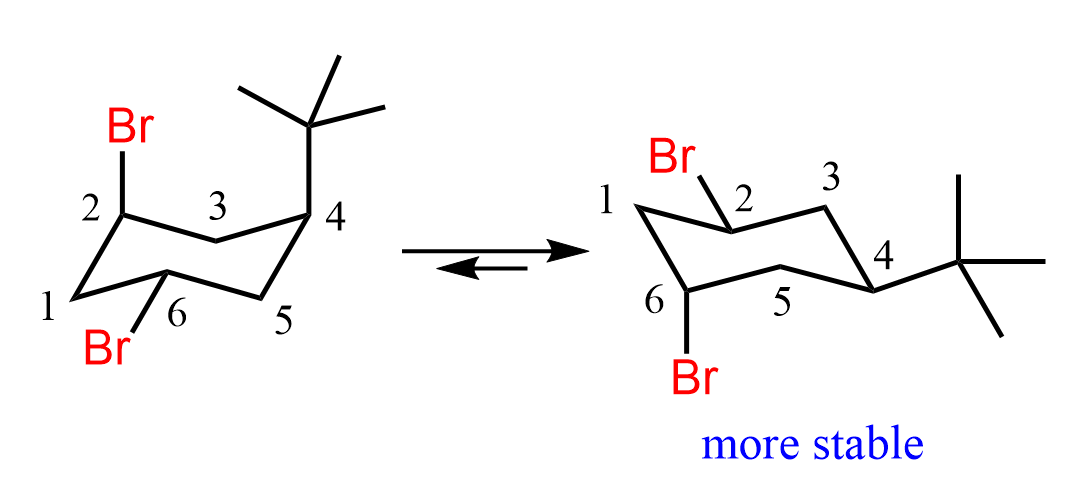
In the first conformation, we have an axial tert-butyl group and an axial bromine, whereas in the second structure, there is only one axial bromine. This means the conformation on the right side is more stable, and the SN2 substitution occurs by the nucleophile expelling the axial bromine (C6).
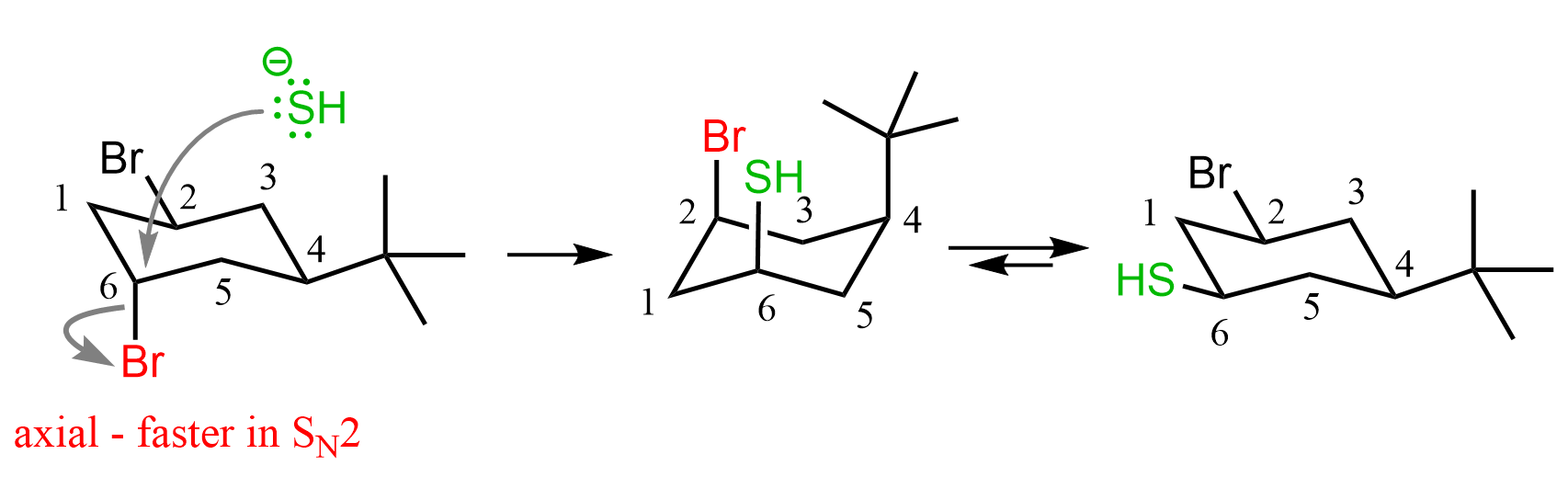
Now, this does not mean that C2-Br in the first conformation (left chair conformer in the ring flip image) does not participate in SN2 substitution at all. It is, in fact, on the same side as the t-butyl group, which means the nucleophile is not hindered so much when attacking from the opposite side. The determining factor is the stability of the chair conformation. The conformation on the right is more stable, which means the molecule is predominantly in this conformation and the corresponding axial bromine is substituted by the nucleophile.
Let’s summarize the steps for determining the major product of SN2 reactions of cyclohexanes, considering the effects of the stability and orientation of substituents on the ring:
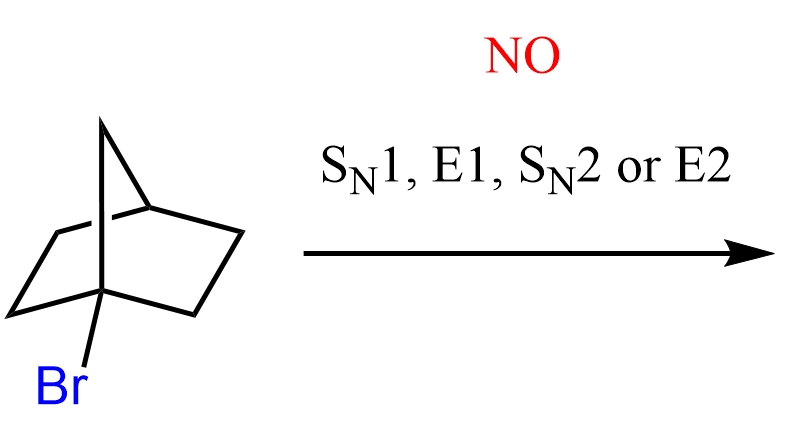
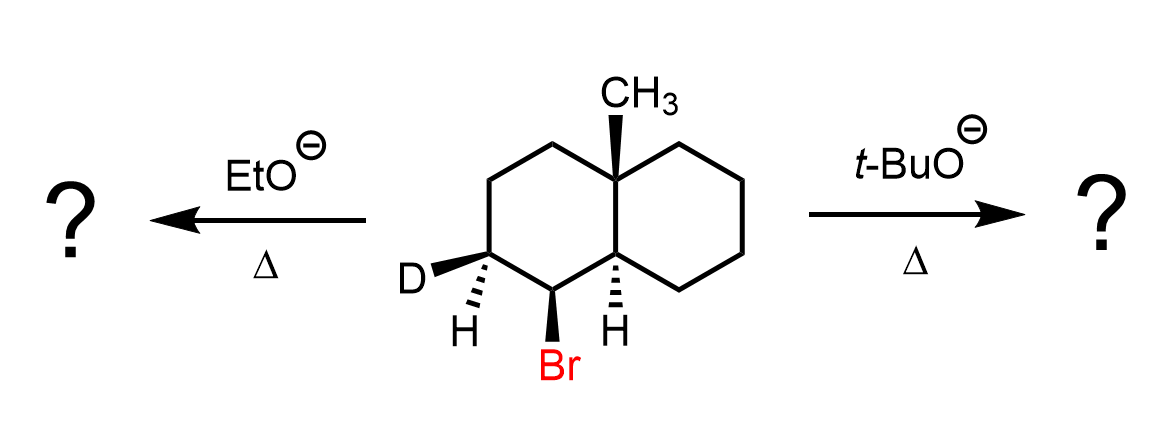
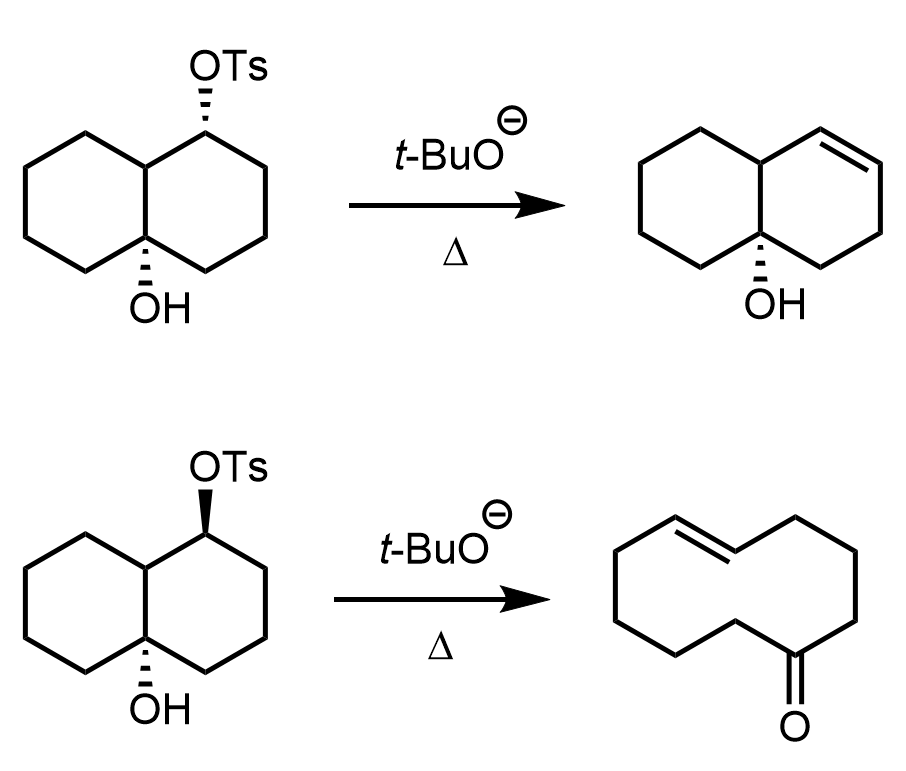
Below are some practice problems emphasizing the importance of orbital alignment in SN2 and E2 reactions.
In both mechanisms, the geometry of the reacting molecule plays a crucial role in determining whether the reaction can occur and how fast it proceeds.
For SN2 reactions, the nucleophile must attack the electrophilic carbon from the backside, directly opposite the leaving group. This alignment allows for the proper overlap between the nucleophile’s filled orbital and the antibonding σ* orbital of the C–X bond, leading to the inversion of configuration known as the Walden inversion.
In E2 reactions, orbital alignment is equally important: the β-hydrogen and the leaving group must be anti-periplanar, meaning they lie in the same plane but on opposite sides of the molecule. This arrangement enables effective overlap of the σ(C–H) and σ*(C–X) orbitals, forming the new π bond in the product alkene. Periplanar arrangement with 0o is also possible, as you will see in the practice question below.

Could you please, cite the references of the reactions mentioned in this article ?
Most organic chemistry textbooks have a touch on this. I did not rely on any research paper to show the examples here, however, Clayden explains this quite nicely (p 464).