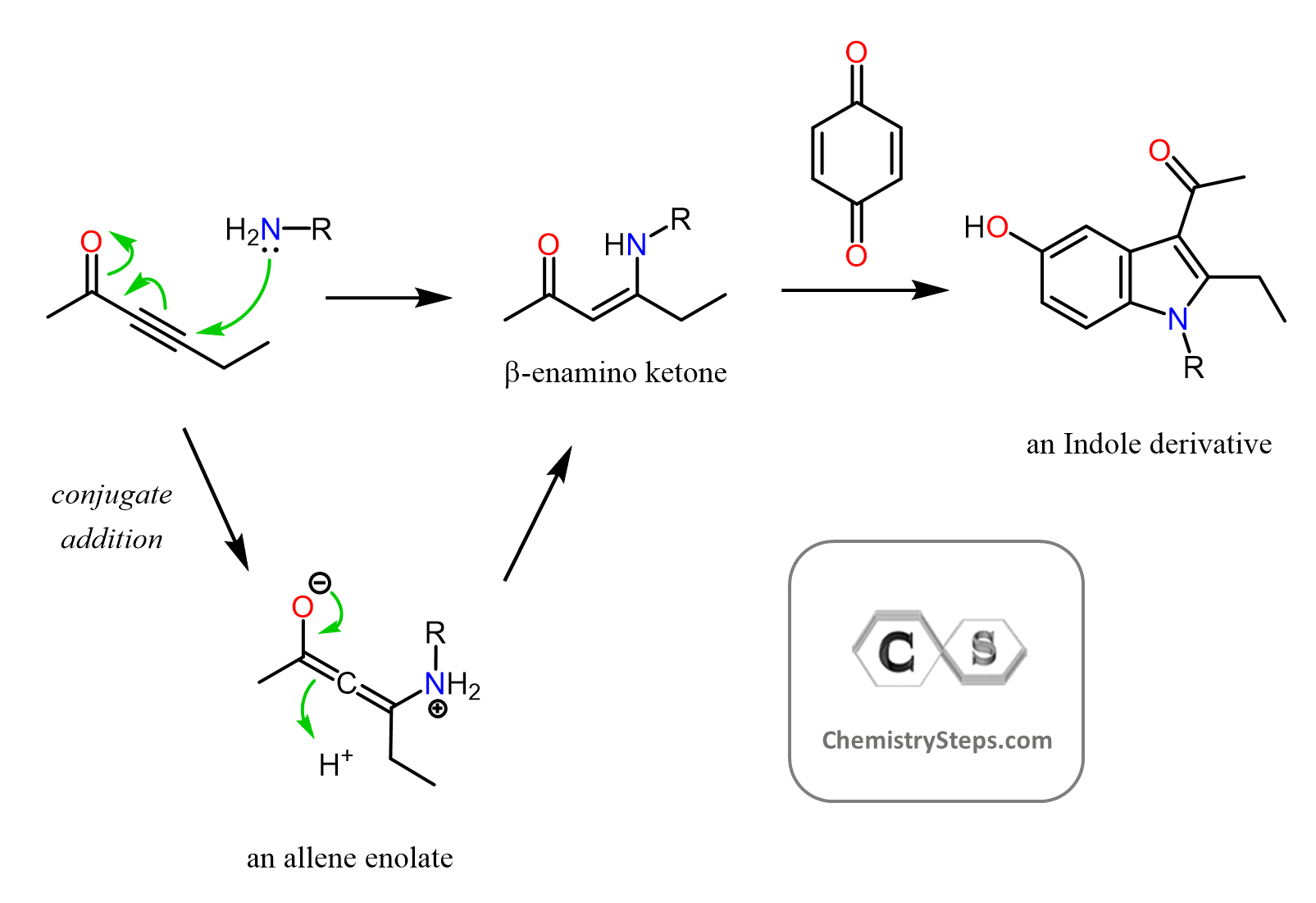
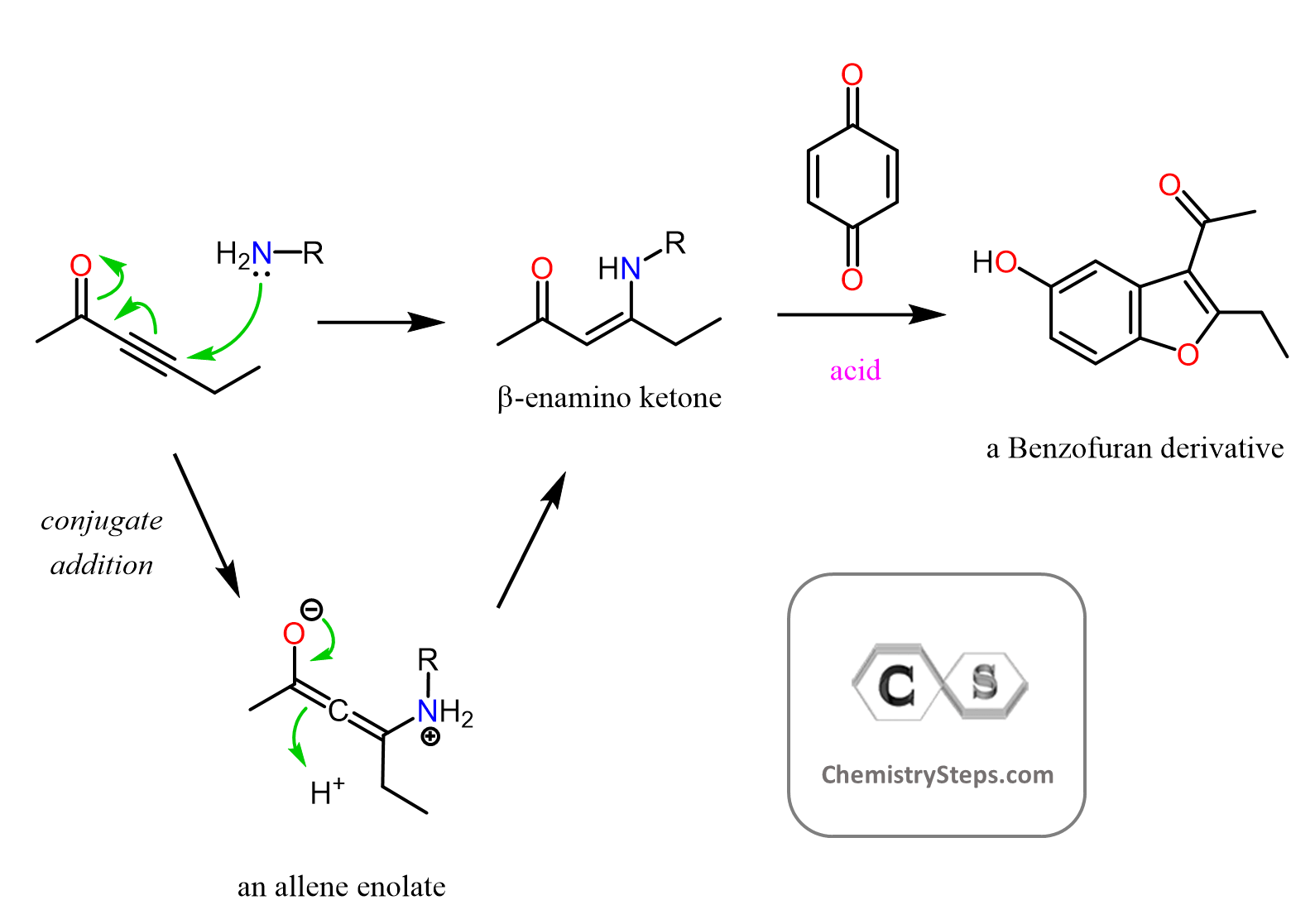
We talked about the Michael reaction in which the nucleophile is a doubly stabilized enolate performing a conjugate (1, 4) addition:
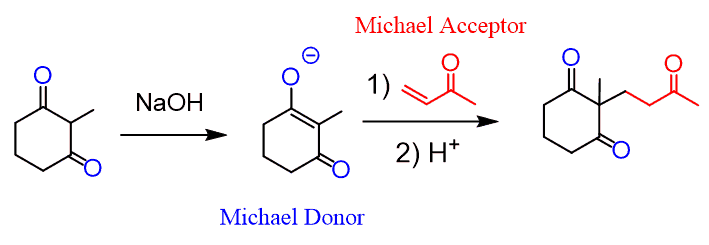
Remember, at the same time, that regular enolates are not efficient Michael donors since they are stronger bases and therefore, prefer the 1, 2-addition mechanism.
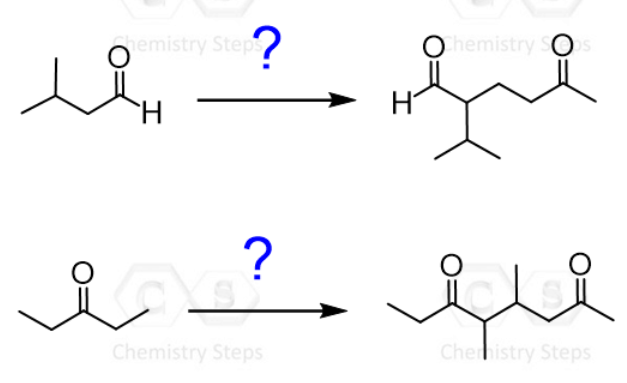
So, taking this into consideration, how would you accomplish the following transformation?
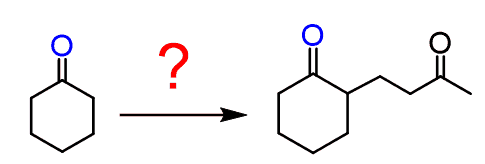
The problem is that we cannot simply make an enolate using LDA since it is not an efficient Michael donor. On the other hand, it is not a dicarbonyl compound either to form a doubly stabilized enolate.
And this is where the Stork enamine synthesis becomes handy. It relies on the nucleophilicity of the double bonds in enamines originating from the electron-donating effect of the lone pairs on the nitrogen.
We can see the nucleophilic sites by drawing the resonance structure of an enamine:
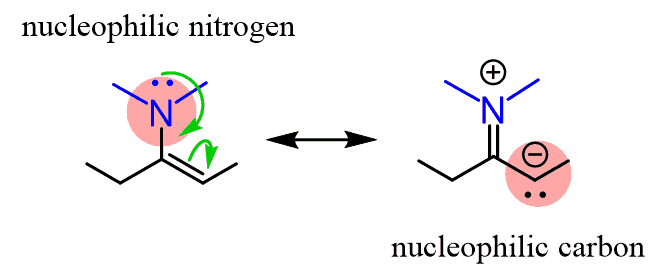
You may wonder what enamines have to do with the ketone and the transformation shown with the question mark.
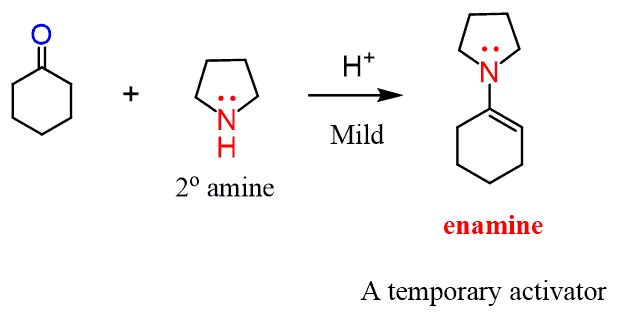
Remember, enamines are the product of reacting aldehydes and ketones with secondary amines:

Both the nitrogen and the carbon are nucleophilic, and the nucleophilic carbon makes the enamines great for alkylation, acylation, and conjugate addition:
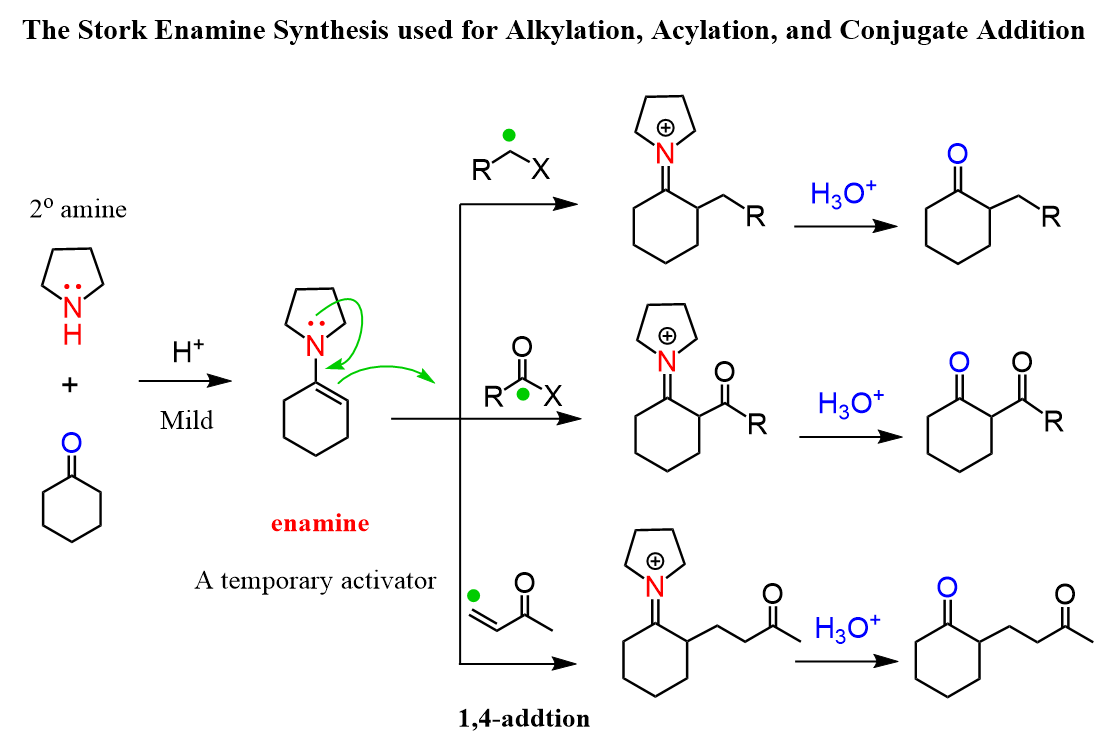
Enamines are great and perhaps a superior alternative to the direct alkylation with the following advantages:
- Milder conditions – No need for strong bases
- Minimize the double alkylation
- And importantly, works for aldehydes as well
The Mechanism of Stork Enamine Synthesis
The first part is the enamine formation, and you can refer to this post for the mechanism of this reaction between aldehydes and ketones with amines:
In the second part, we have the nucleophilic attack of the enamine. For the alkylation, it is a simple SN2 mechanism, the acylation is an addition-elimination mechanism, and the conjugate addition is what we saw in the Michael reaction:
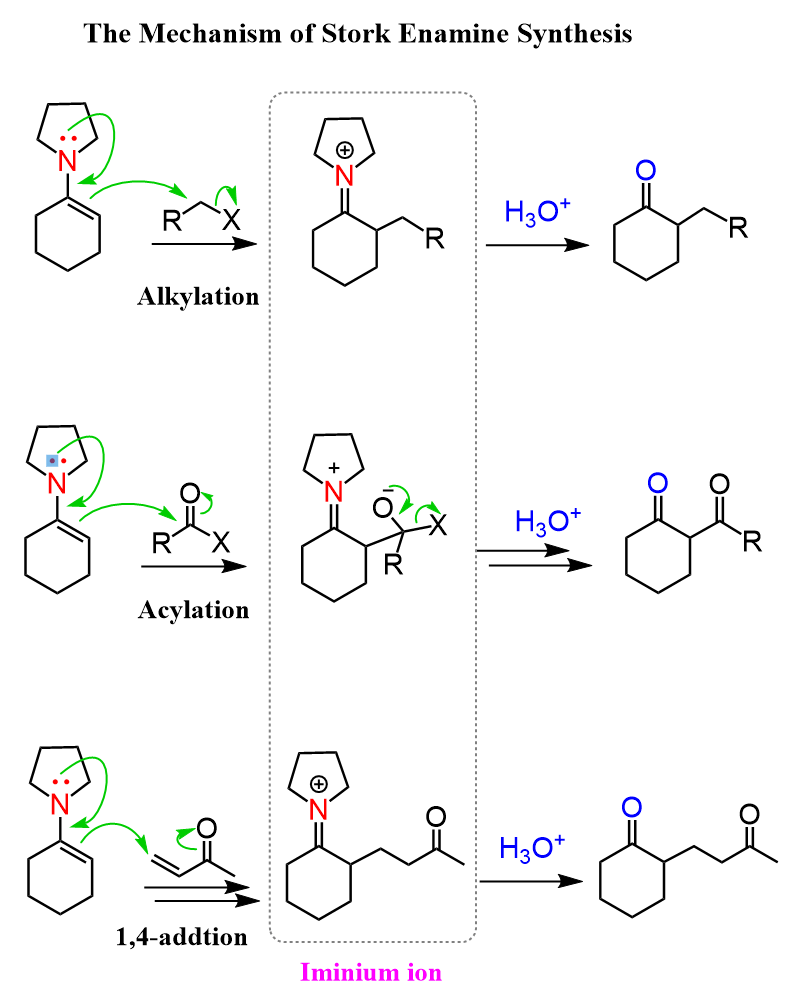
Notice that in all the reactions, there is an iminium ion intermediate, which is hydrolyzed back to the carbonyl under acidic conditions.
So, overall, the enamine serves as a temporary activator by turning the carbonyl into a nucleophile.
The main problem here can be the competing reaction of the nitrogen:
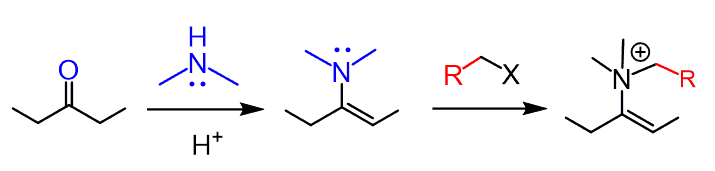

One of your diagrams has an acyl halide reacting with an enamine to produce an alkylated cyclohexanone. Then the next diagram has an alkyl halide reacting to produce an acylated cyclohexanone.
Thank you so much for letting me know! Fixed.