The acid-base reactions are very important in organic chemistry as they lay the foundation of many principles used in other chapters, such as resonance stabilization, substitution, elimination reactions, and many more.
One of the key skills in acid-base chemistry is understanding the pKa table and being able to use it to predict the outcome of an acid-base reaction.
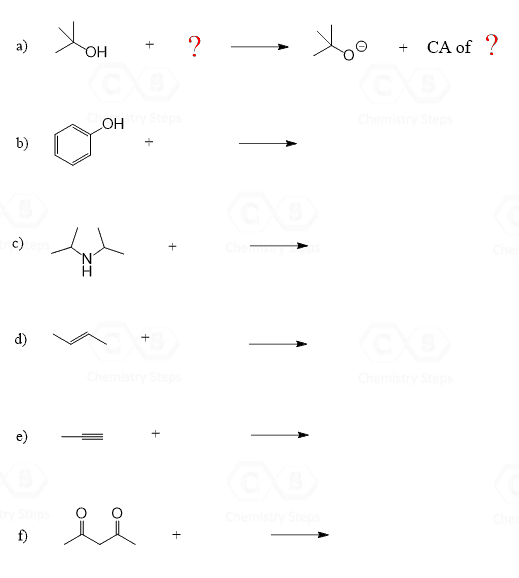
Let’s say you are given the following compound (phenol) and asked to deprotonate it:
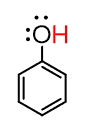
First of all, deprotonation means removing the most acidic proton of the compound by a base that you need to choose. We call it a base because if the given compound is deprotonated, then it is a proton donor, and by Brønsted–Lowry definition, the proton donor is the acid in an acid-base reaction. So, we can visualize the task as such, we need something (a base) to react with the phenol and remove the red H:
And how do we find this base?
The principle that you need to rely on to find a proper base is that any acid-base reaction lies to the side of forming a weaker acid and a base.
Remember, a strong acid and a base react to form a weak acid and a base.
And because the acid strength is quantified by the pKa value, we need to identify the pKa of the acid and the conjugate acid (on the right side) of the reaction to determine which side the equilibrium will shift.
So, to start with, we are going to identify the pKa of the compound that we need to deprotonate. In this case, it is the phenol with pKa = 10.
Next, we can react this with a hypothetical base, abbreviated as B–. In the products, we are going to have the deprotonated phenol (the conjugate base of the phenol), and the protonated B, shown as B-H, which is the conjugate acid of this base:
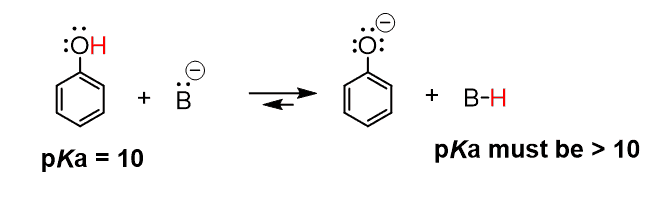
The equilibrium of this reaction needs to be shifted to the right side in order for us to say that B is a correct choice as a base to deprotonate phenol. This means that the B-H has to have a higher pKa value (weaker acid) than phenol.
At this point, look up in the table to find a compound with a pKa > 10 and put it in place of the B-H.
There are quite a lot of options, and we can pick any of them. For example, we will pick the alcohol and use ethanol on the product’s side.

This means that the B– should be the conjugate base of the ethanol. So ethoxide (with a counter ion) can be used to deprotonate the phenol. Let’s write up the complete equation then:
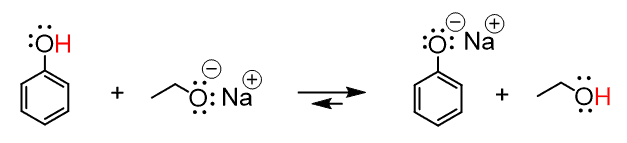
The sodium here is a counterion, which is most often not important in organic reactions, so the equation can also be shown without it:
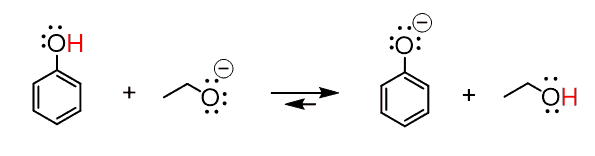
So, to generalize this, if you need to choose a base to deprotonate a compound that has, for example, a pKa = 10, you can pick anything from the pKa table that has a pKa > 10 and use its conjugate base.
Remember, the weaker the acid, the stronger the conjugate base:

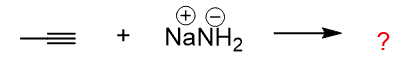
As an example: Can sodium amide deprotonate the following alkyne?
The hydrocarbons are generally considered very weak acids, but among them, the alkynes, with a pKa = 25, are quite acidic. This means the most acidic proton in this molecule is on the terminal alkyne (sp C-H).
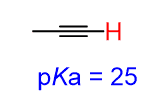
To find out whether the sodium amide can deprotonate the alkyne, we need to first identify the conjugate acid of the amide by adding a proton to it:
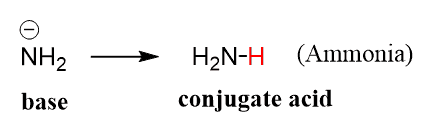
Ammonia is the conjugate acid of the base, so now, we can use the pKa table to write the acid-base reaction with the pKa value of ammonia. Ammonia is an amine, and amines have a pKa ~ 38, so the reaction goes from pKa ~ 25 to pKa ~ 38, which is a favorable pKa change and that is why this reaction would work:
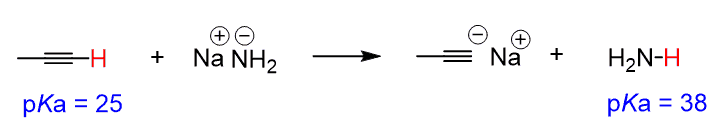
Looking at the pKa chart, you can see that the conjugate bases of alkanes and alkenes would also work to deprotonate the alkyne to form what is called acetylide ions.
The deprotonation of terminal alkynes is an important reaction in organic chemistry, as it allows for the creation of a carbon nucleophile that can participate in SN2 substitution reactions with various alkyl halides and epoxides.
Now, let’s learn how to choose a suitable acid for protonating a given compound.
How to choose an acid to protonate a given compound
In this case, as well, we are going to follow the main principle of acid-base reactions is that we need to choose a compound such that the reaction produces a weaker acid (and a base), i.e. higher pKa value.
For example:
Compound A is an intermediate in a Grignard reaction (a common reaction in organic chemistry). Choose a compound from the pKa table to protonate this alkoxide ion:
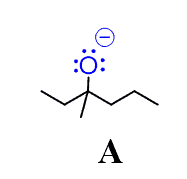
First, let’s write down the equation for this protonation reaction. We will use a hypothetical acid (A-H) to achieve this:

One of the products on the right side is the protonated form (conjugate acid) of the alkoxide, which is an alcohol. The product in this reaction is a 3o (tertiary) alcohol, which is less acidic and is at the higher end of the alcohol pKa range (16-18). An appropriate reagent for the protonation would be one with a pKa lower than 18.
So, the A-H can be anything with a pKa < 18. For example, water can be used to protonate this intermediate:
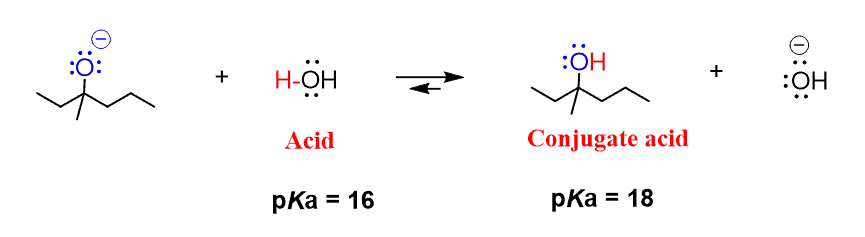
Other options, in theory, can be phenol, acetic acid, and all the inorganic acids such as HCl, H2SO4 and etc. However, in practice, not every acid-base reaction is suitable to carry out in a laboratory because these are one of the fastest and exothermic reactions, and reactions involving very strong acids with very strong bases are often dangerous, and the other factor is, of course, the pricing of the chemicals.
Summary: Protonating and Deprotonating a Compound
To summarize, everything related to acid-base reactions can be, and is, explained by the pKa values (and pKb for bases) of the acids. Choosing a proper base or an acid is no exception, and when doing it, you need to keep in mind that the acid-base equilibrium is shifted to the weak acid (higher) pKa and base formation.
To find a suitable acid, remember, for example, that any compound with a lower pKa value (stronger acid) can protonate another compound whose conjugate acid has a higher pKa value.
Example:

Any base with a conjugate acid having a higher pKa value (weaker acid) can deprotonate another compound.
Example:

You’ll see this pattern play out in many important reactions, especially nucleophilic substitution and elimination. In those mechanisms, acid-base steps often control what is a good nucleophile, a strong base, and whether the given moiety can serve as a leaving group.
So, being able to read a pKa table and make these comparisons is a core skill you’ll rely on throughout your organic chemistry course.
Here is also a list of the pKa values for the most common functional groups that you can use to solve the following practice problems: