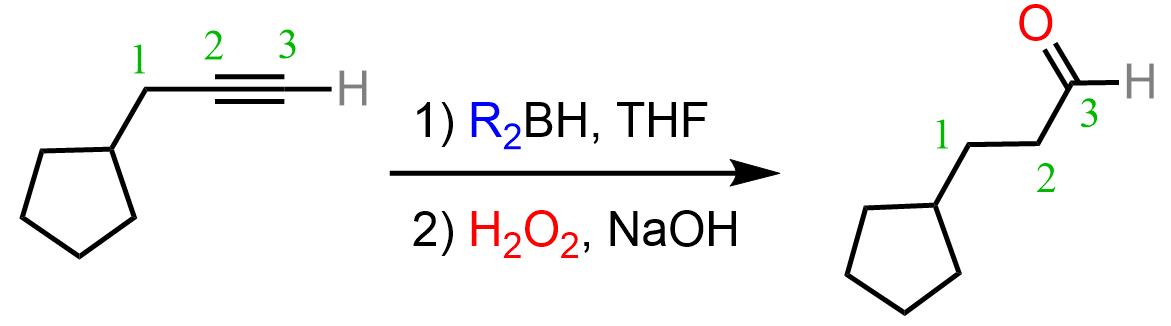
Hydroboration-Oxidation of Alkynes
Like alkenes, alkynes can also be subjected to Hydroboration-Oxidation to achieve an anti-Markovnikov addition of an OH group to the π bond. As in the case of acid-catalyzed hydration and oxymercuration, the intermediate here is an enol, which, this time, tautomerizes into an aldehyde as the OH group is on the terminal carbon atom:

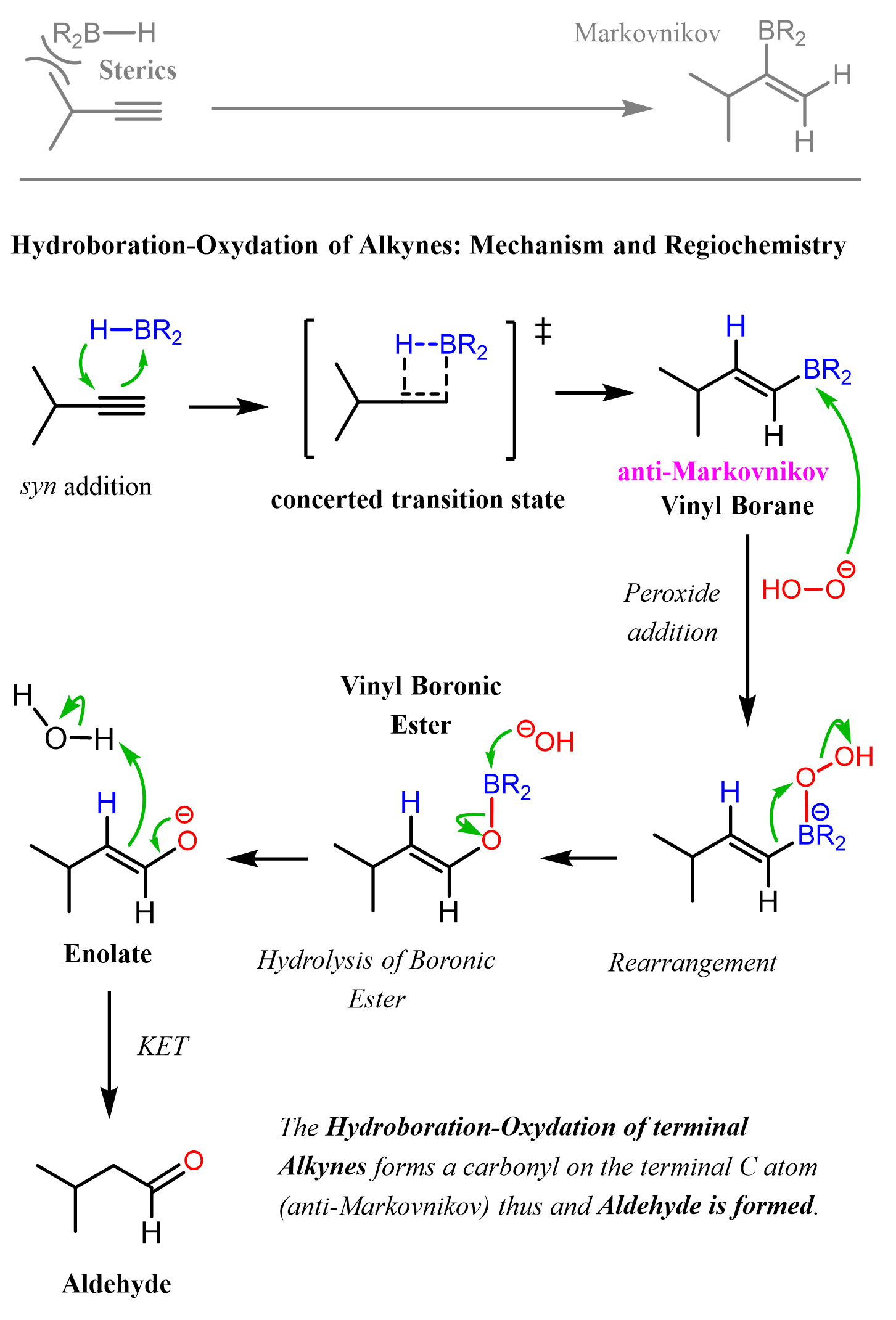
Because alkynes have two π bonds, diallyl boranes (R2BH) are often used to prevent the second addition of the boron to the boron-substituted alkene.
There is a separate article about enols and keto-enol tautomerization that you can read, but let’s quickly mention what enols are and why they are not particularly stable.
An enol is a molecule that combines an alkene (ene) and alcohol (ol):

We already know that alkenes are electron-rich, and it turns out that having an OH group on them increases the electron density even more via resonance donation. As a result, enols are very reactive and they interconvert to ketones or aldehydes on their own. You can view KET as a proton transfer from the OH group to the C=C double bond. This converts the C-OH into a C=O, and the C=C into a C-C bond:
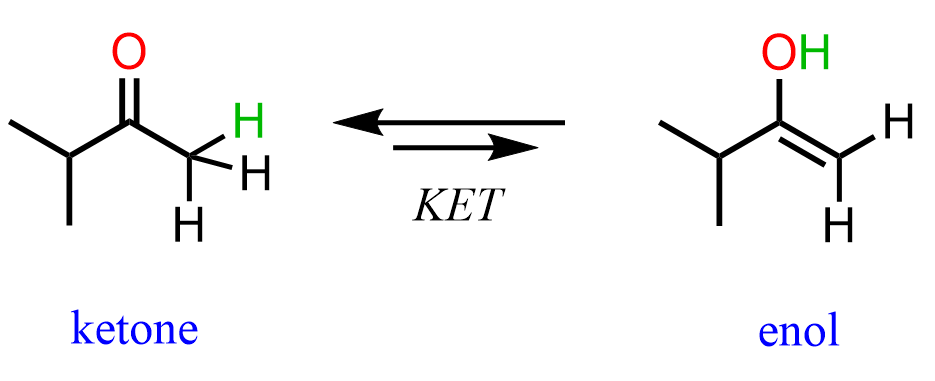
Notice that ketones and enols are different compounds that are in equilibrium, and thus we show keto-enol tautomerization with a double arrow. Do not mix this with resonance structures, which are just different representations of the same compound shown with a double-headed arrow. Aldehydes and ketones are constitutional isomers of their corresponding enols.
The Mechanism of Hydroboration-Oxidation of Alkynes
The reaction starts with a concerted addition of the alkylborane to the triple bond, forming a vinyl borane. Notice that this process is regioselective and due to the steric hindrance of the alkyl groups on the boron and the triple bond, which favors the opposite orientation, which adds the boron to the less substituted carbon atom (anti-Markovnikov addition). In the subsequent steps of oxidation and rearrangement, the BR2 group is replaced with an OH group, which, after a keto-enol tautomerization, forms the final product, aldehyde:
The hydroboration-oxidation of terminal alkyne gives a mixture of ketones. Depending on the difference in the bulkiness of alkyl groups, one of the ketones may form in excess.
The Regioselectivity of Hydroboration-Oxidation of Alkynes
Notice that in the first step of the hydroboration mechanism, we have a concerted addition of the alkyl borane to the alkyne. The radiochemical outcome of the reaction is defined by the relative orientation of the alkyne and the alkyl borane. Because of steric interactions, the alkyl groups on the boron and the triple bond are oriented oppositely, which adds the boron to the terminal carbon. This is the “seed of growing an anti-Markovnikov carbonylation” of internal alkynes. The resulting vinyl borane is oxidized to the corresponding enol, which eventually gives the final product aldehyde.
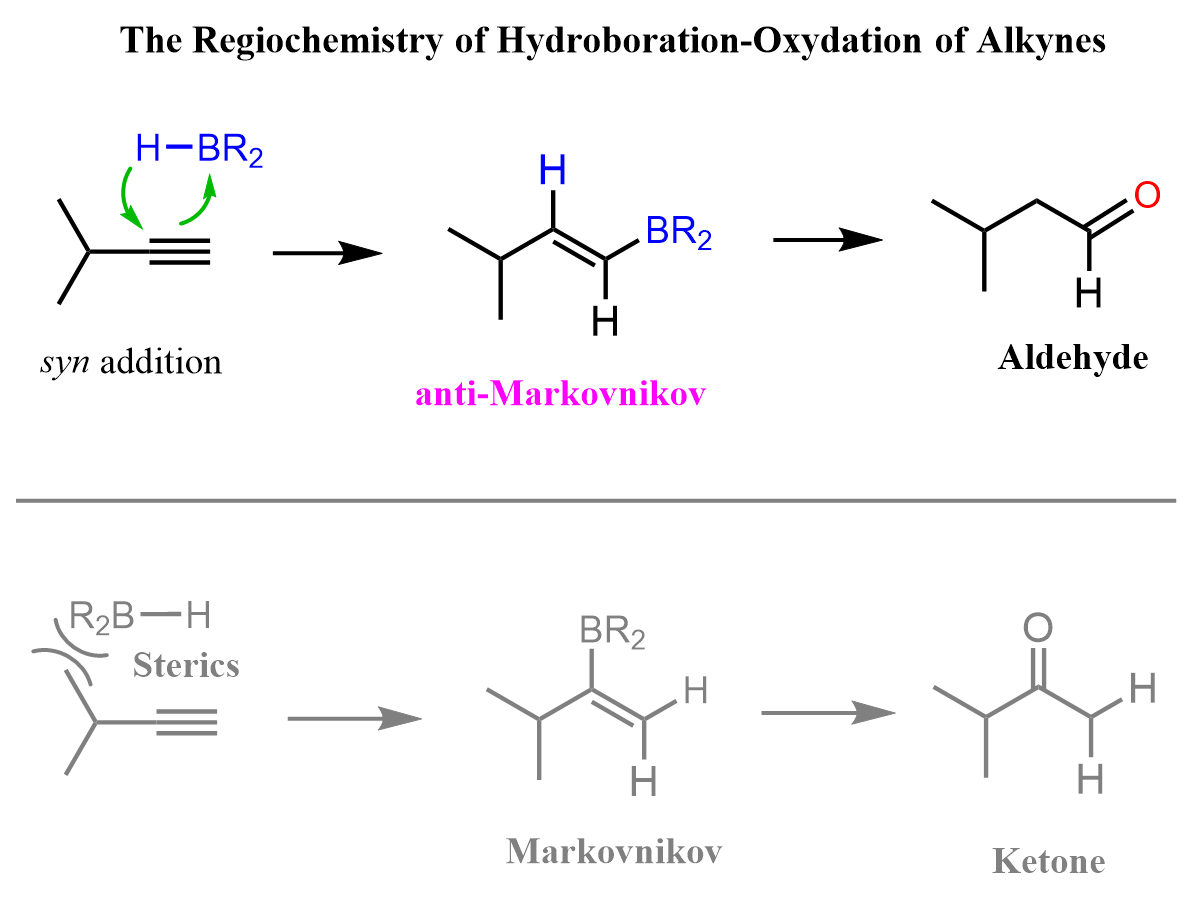
To enhance the steric interaction and thus the regioselectivity of the reaction, bulky alkyl boranes such as 9-BBN and Disiamylborane (Sia2BH) are used instead of borane, BH3, which we have seen in the hydroboration-oxidation of alkenes:
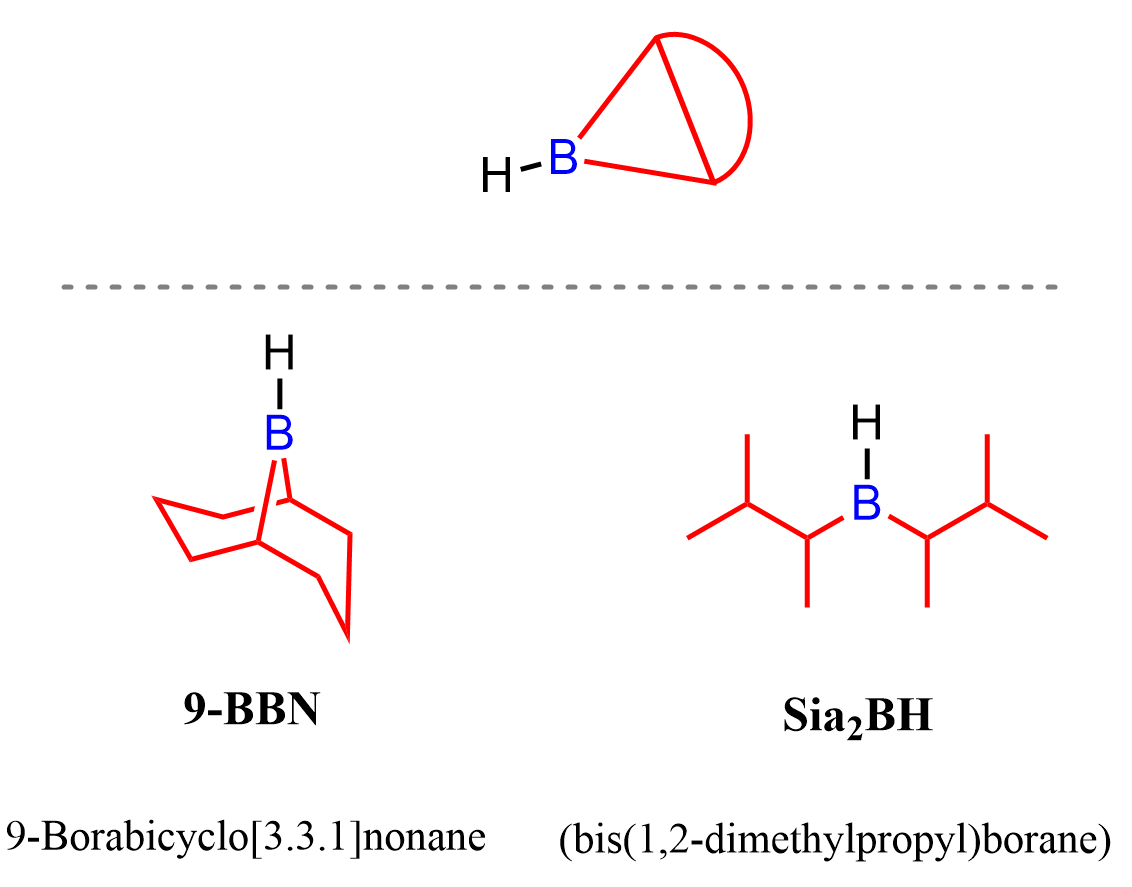
The bulky nature of 9-BBN is especially handy for achieving anti-Markovnikov addition when terminal alkynes are used:

Both hydroboration-oxidation and acid-catalyzed hydration of Internal alkynes produce a mixture of ketones, as there is no less substituted carbon and boron added to both carbons of the triple bond in an equal amount. Symmetrical alkynes can only give one ketone regardless of the hydration reaction:

Below are some practice problems on the hydroboration-oxidation of alkenes, and you can find more on this and other methods of hydration of alkynes in this article.

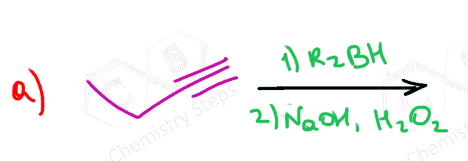


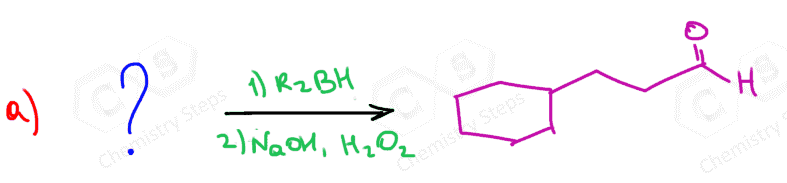
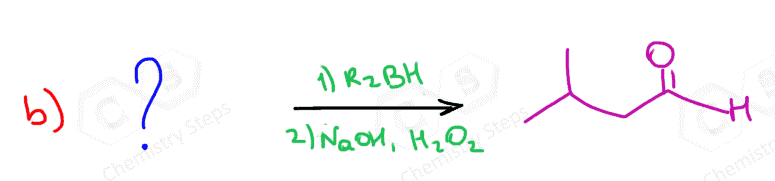
Very helpful..