ChatGPT said:
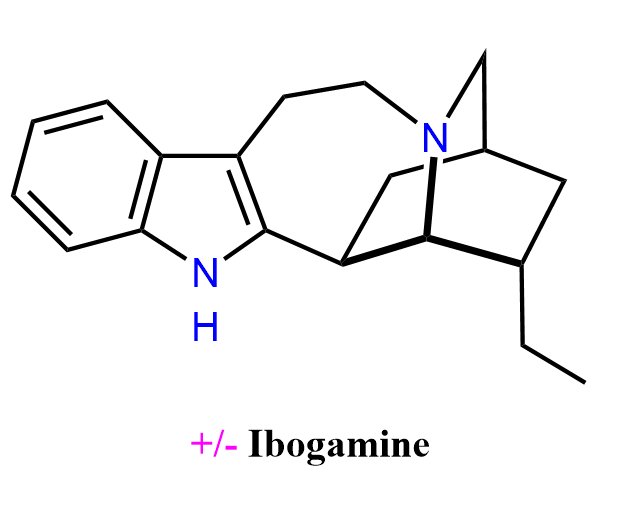 Ibogamine is a naturally occurring iboga-type indole alkaloid found in Tabernanthe iboga and related species, and belongs to the same structural family as ibogaine, tabernanthine, and coronaridine. Like other iboga alkaloids, ibogamine has been studied for its psychoactive and potential anti-addictive properties, although it is not an approved medication in any major jurisdiction. Animal studies indicate that iboga alkaloids, as a class, can reduce morphine and cocaine self-administration, largely through modulation of dopamine release in the nucleus accumbens, although the specific contribution of ibogamine is less well characterized. Importantly, early pharmacology data show that both R- and S-ibogamine lack the tremorigenic neurotoxicity seen with other iboga compounds such as ibogaine and harmaline, making ibogamine one of the non-tremorigenic members of the series. Despite this, ibogamine still shares the broader concerns associated with iboga alkaloids, including potential neurotoxicity and significant safety risks, and it has no established clinical use or regulatory approval. Its relevance today is primarily in chemical, pharmacological, and structure–activity studies of the iboga scaffold.
Ibogamine is a naturally occurring iboga-type indole alkaloid found in Tabernanthe iboga and related species, and belongs to the same structural family as ibogaine, tabernanthine, and coronaridine. Like other iboga alkaloids, ibogamine has been studied for its psychoactive and potential anti-addictive properties, although it is not an approved medication in any major jurisdiction. Animal studies indicate that iboga alkaloids, as a class, can reduce morphine and cocaine self-administration, largely through modulation of dopamine release in the nucleus accumbens, although the specific contribution of ibogamine is less well characterized. Importantly, early pharmacology data show that both R- and S-ibogamine lack the tremorigenic neurotoxicity seen with other iboga compounds such as ibogaine and harmaline, making ibogamine one of the non-tremorigenic members of the series. Despite this, ibogamine still shares the broader concerns associated with iboga alkaloids, including potential neurotoxicity and significant safety risks, and it has no established clinical use or regulatory approval. Its relevance today is primarily in chemical, pharmacological, and structure–activity studies of the iboga scaffold.
Practice
Add the missing reagents and intermediates in the synthesis of Ibogamine and draw a plausible mechanism for the conversion from intermediates 2 to 6.
This content is for registered users only.
Click here to Register!
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems, including over 40 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, Reaction Maps, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.

 Ibogamine is a naturally occurring iboga-type indole alkaloid found in Tabernanthe iboga and related species, and belongs to the same structural family as ibogaine, tabernanthine, and coronaridine. Like other iboga alkaloids, ibogamine has been studied for its psychoactive and potential anti-addictive properties, although it is not an approved medication in any major jurisdiction. Animal studies indicate that iboga alkaloids, as a class, can reduce morphine and cocaine self-administration, largely through modulation of dopamine release in the nucleus accumbens, although the specific contribution of ibogamine is less well characterized. Importantly, early pharmacology data show that both R- and S-ibogamine lack the tremorigenic neurotoxicity seen with other iboga compounds such as ibogaine and harmaline, making ibogamine one of the non-tremorigenic members of the series. Despite this, ibogamine still shares the broader concerns associated with iboga alkaloids, including potential neurotoxicity and significant safety risks, and it has no established clinical use or regulatory approval. Its relevance today is primarily in chemical, pharmacological, and structure–activity studies of the iboga scaffold.
Ibogamine is a naturally occurring iboga-type indole alkaloid found in Tabernanthe iboga and related species, and belongs to the same structural family as ibogaine, tabernanthine, and coronaridine. Like other iboga alkaloids, ibogamine has been studied for its psychoactive and potential anti-addictive properties, although it is not an approved medication in any major jurisdiction. Animal studies indicate that iboga alkaloids, as a class, can reduce morphine and cocaine self-administration, largely through modulation of dopamine release in the nucleus accumbens, although the specific contribution of ibogamine is less well characterized. Importantly, early pharmacology data show that both R- and S-ibogamine lack the tremorigenic neurotoxicity seen with other iboga compounds such as ibogaine and harmaline, making ibogamine one of the non-tremorigenic members of the series. Despite this, ibogamine still shares the broader concerns associated with iboga alkaloids, including potential neurotoxicity and significant safety risks, and it has no established clinical use or regulatory approval. Its relevance today is primarily in chemical, pharmacological, and structure–activity studies of the iboga scaffold.