In the previous post, we talked about the Hofmann reaction where amines are converted into quaternary ammonium salts and later to alkenes. Remember, amines, and especially the conjugate bases of amines, are strong bases and therefore, they are poor leaving group and cannot participate in an elimination reaction. Quaternary ammonium salts, on the other hand, are good leaving group since the departing nitrogen is charge-free and with a complete octet.
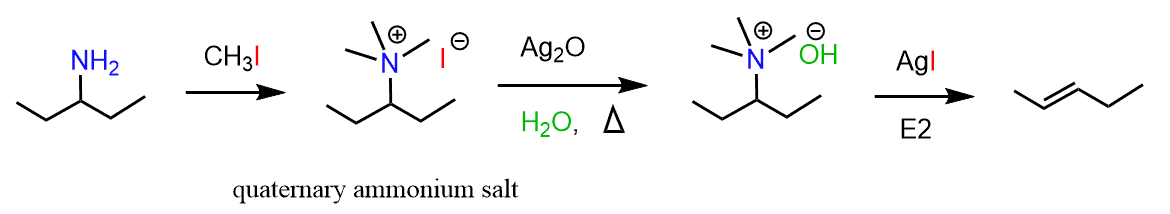
The Cope elimination is conceptually similar to this and occurs when tertiary amines are treated with hydrogen peroxide and then heated up.
What happens here is the amine first reacts with the peroxide which oxidizes it to a tertiary amine oxide. This oxide now is a strong base and a good leaving group on the form of a hydroxyl amine. So when heated, the amine oxide then undergoes elimination forming an alkene and a N,N-dialkylhydroxylamine:

The elimination proceeds via a five-membered cyclic transition state and it is a one-step, concerted internal elimination during which, the amine oxide serves both as a base and a leaving group.
These types of reactions are also referred to as Ei (elimination intramolecular). In general, the reaction is unimolecular and follows the same rate law as the E1 elimination:

However, an important difference between the Cope elimination and the E1 and E2 mechanisms is its stereochemistry.
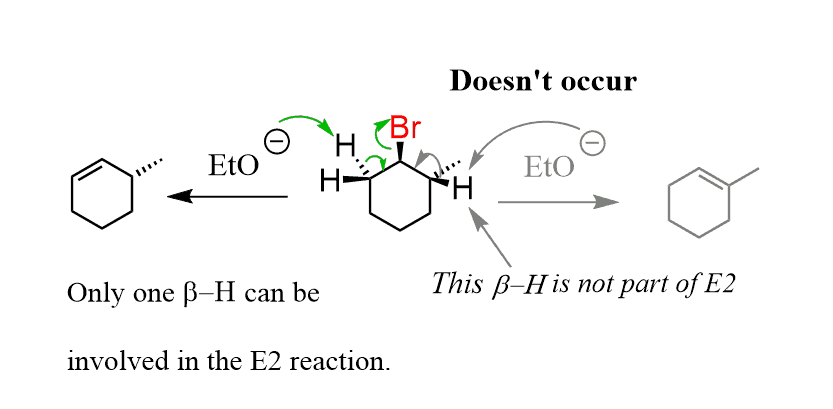
Remember, in the conventional elimination reactions, the β proton and the leaving group needed to be in an anti-periplanar orientation to support the necessary orbital overlap:
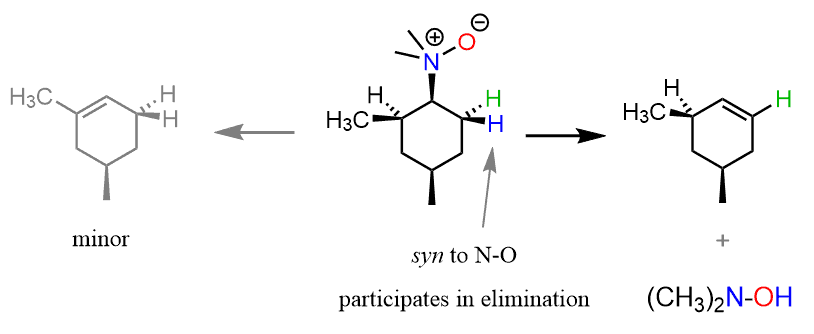
In the Cope elimination, the β hydrogen and the leaving group are in a syn periplanar orientation since it is the only conformation that allows the oxygen to attack the proton. For example, the following Cope elimination of a cyclohexyldimethylamine produces one alkene as the major product dictated by the syn stereoselectivity:
Notice that the other regioisomer would have been formed if an analogous halogen derivative was used in the presence of a strong, bulky base because of the anti-periplanar orientation between the β hydrogen and the leaving group:
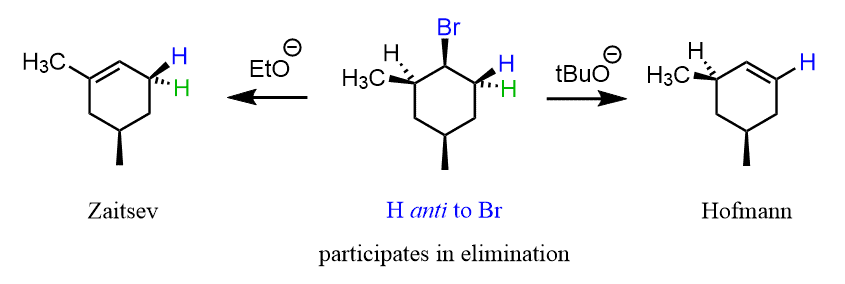
In general, the regiochemistry of the Cope elimination does not follow either the Zaitsev or Hofmann path, and depends on the presence of hydrogen atoms at the β-positions capable of adopting syn geometry.
There are analogous reactions of esters and xanthates (Chugaev reaction) which proceed via six-membered cyclic transition states:
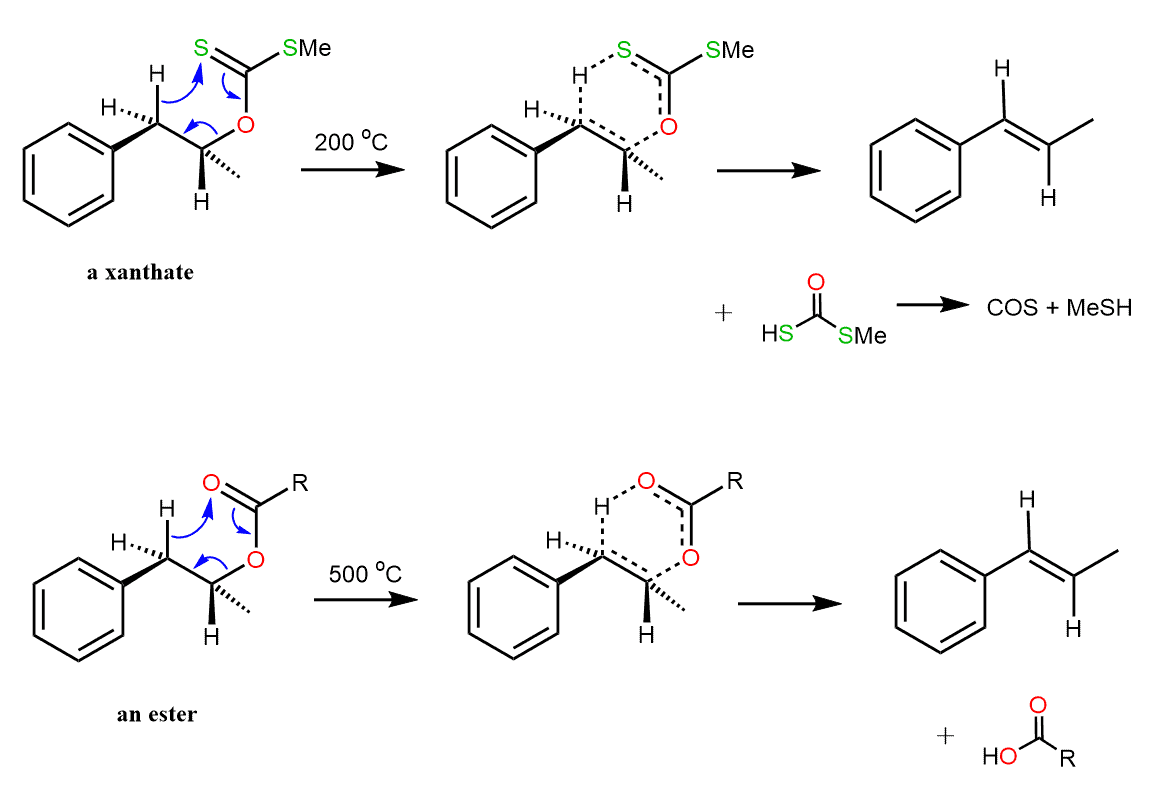
The transition state in these reactions is not as planar and therefore, the syn stereoselectivity is less pronounced. In both cases, the reaction generally requires a higher temperature than the Cope elimination.
One of the advantages of these reactions is that they don’t require harsh conditions such as the use of concentrated sulfuric acids, which is used in the dehydration of alcohols. Aside from the conditions, remember that reactions going via carbocation formation might be prone to rearrangements, while concerted reactions are safer in this sense. For example, the following alcohol can be transformed into an alkene using the Chagaev reaction, where it is first converted into a xanthate:
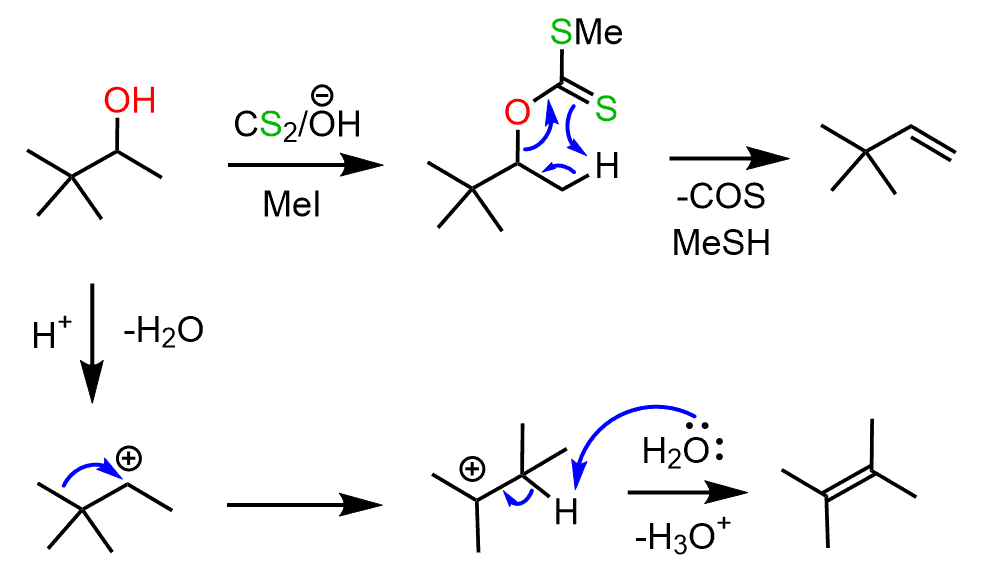
Notice that if dehydration was used, the desired alkene wouldn’t have only been the minor product because of the rearrangement of the secondary carbocation.
Check Also
- Naming Amines: Systematic and Common Nomenclature
- Preparation of Amines
- The Gabriel Synthesis of Primary Amines
- The Hofmann Elimination of Amines and Alkyl Fluorides
- Imines from Aldehydes and Ketones with Primary Amines
- Enamines from Aldehydes and Ketones with Secondary Amines
- The Reaction of Amines with Nitrous Acid
- Reactions of Amines Practice Problems
