Nitriles can be hydrolyzed to carboxylic acids in acidic aqueous solutions, and to carboxylate salts with base-catalyzed hydrolysis:

In both cases, the transformation consists of two main parts; conversion of the nitrile to an amide and hydrolysis of the amide to the corresponding carboxylic acid.
The Mechanism of Acid-Catalyzed Nitrile Hydrolysis
In the first step of acid-catalyzed hydrolysis, the protonation of the nitrogen activates the C-N triple bond for a nucleophilic attack of water:

After deprotonation, a tautomer of an amide called an imidic acid, is formed. An imidic acid is the nitrogen analog of an enol and just like the keto-enol tautomerization, the more stable amide tautomer predominates the equilibrium:
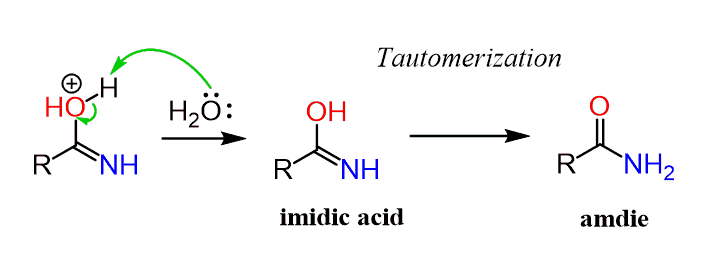
The tautomerization mechanism can be shown in two proton-transfer steps starting with a protonation of the nitrogen:

In the second part, the amide is hydrolyzed to a carboxylic acid by the mechanism we discussed earlier:

The Mechanism of Base-Catalyzed Nitrile Hydrolysis
The base-catalyzed nitrile hydrolysis starts with a nucleophilic addition of the hydroxide ion to the C-N triple bond forming an intermediate with a negative charge on the nitrogen which quickly remove a proton transforming into an imidic:
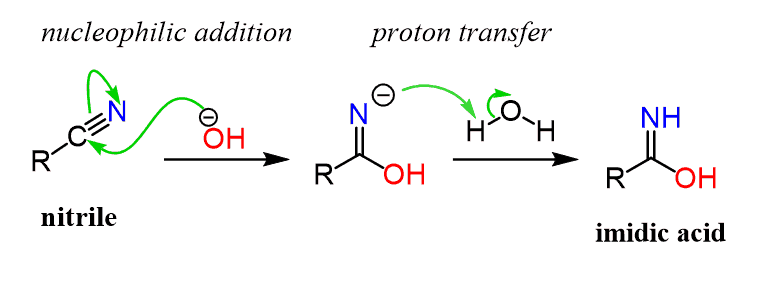
The imidic acid is then deprotonated by the base and after another proton transfer, the amide intermediate is formed.
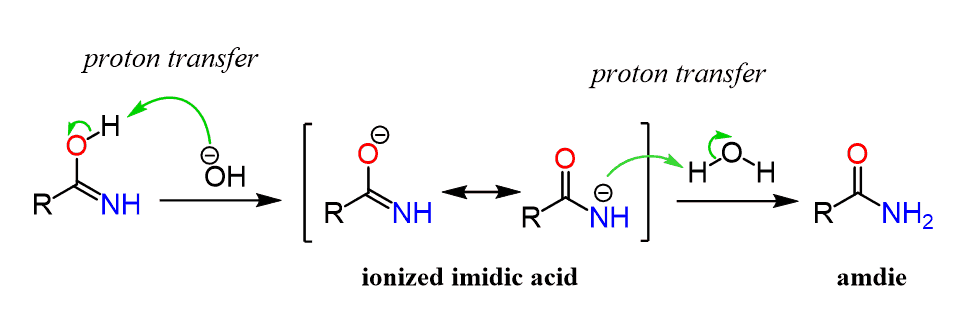
Again, we talked about the hydrolysis of amides earlier, and the summary of the mechanism is combined with the first part of nitrile hydrolysis below:

Need some good practice on the reactions of carboxylic acids and their derivatives?
Check this 45-question, Multiple-Choice Quiz with a 50-min Video Solution covering the reactions of acids, esters, lactones, amides, acid chlorides and etc.
Carboxylic Acids and Their Derivatives Quiz
Check Also
- Preparation of Carboxylic Acids
- Naming Carboxylic Acids
- Naming Nitriles
- Naming Esters
- Naming Carboxylic Acid Derivatives – Practice Problems
- Fischer Esterification
- Ester Hydrolysis by Acid and Base-Catalyzed Hydrolysis
- What is Transesterification?
- Esters Reaction with Amines – The Aminolysis Mechanism
- Ester Reactions Summary and Practice Problems
- Preparation of Acyl (Acid) Chlorides (ROCl)
- Reactions of Acid Chlorides (ROCl) with Nucleophiles
- R2CuLi Organocuprates – Gilman Reagent
- Reaction of Acyl Chlorides with Grignard and Gilman (Organocuprate) Reagents
- Reduction of Acyl Chlorides by LiAlH4, NaBH4, and LiAl(OtBu)3H
- Preparation and Reaction Mechanism of Carboxylic Anhydrides
- Amides – Structure and Reactivity
- Naming Amides
- Amides Hydrolysis: Acid and Base-Catalyzed Mechanism
- Amide Dehydration Mechanism by SOCl2, POCl3, and P2O5
- Amide Reduction Mechanism by LiAlH4
- Reduction of Amides to Amines and Aldehydes
- Amides Preparation and Reactions Summary
- Amides from Carboxylic Acids-DCC and EDC Coupling
- Nitrile Reduction Mechanism with LiAlH4 and DIBAL to Amine or Aldehyde
- The Mechanism of Grignard and Organolithium Reactions with Nitriles
- The Reactions of Nitriles
- Converting Nitriles to Amides
- Carboxylic Acids to Ketones
- Esters to Ketones
- Carboxylic Acids and Their Derivatives Practice Problems
- Carboxylic Acids and Their Derivatives Quiz
- Reactions Map of Carboxylic Acid Derivatives


nice!
Great job
I really like such people who whose work is coherent and we can easily understand it than other similar notes.
Thanks for sharing on the net