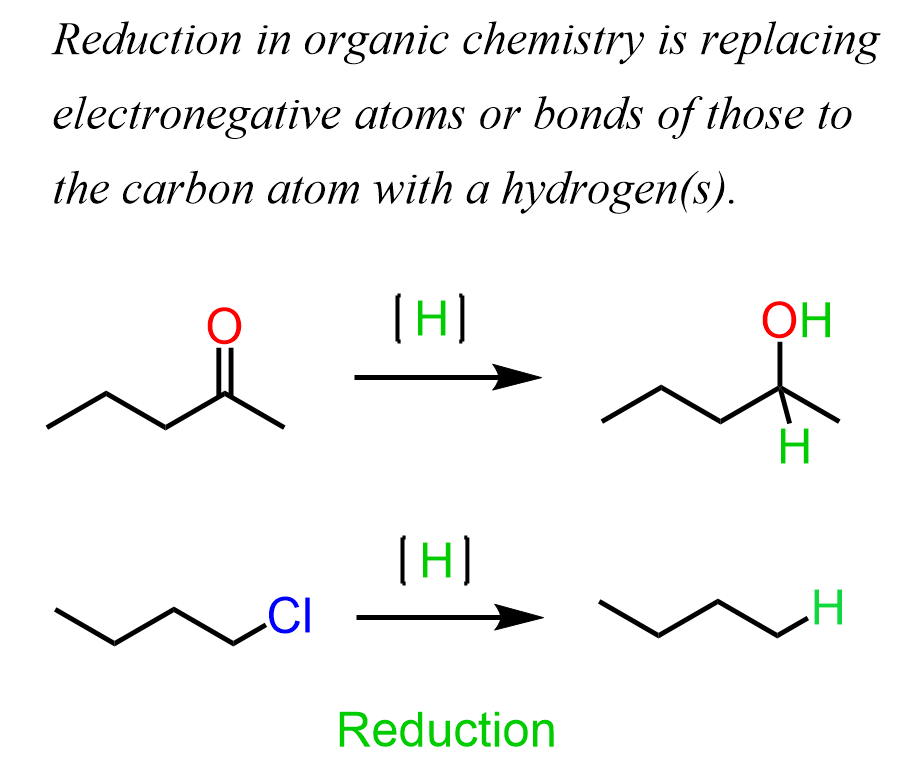
Aldehydes and ketones are carbonyl compounds, and the carbon here is in a relatively high oxidation state:

Notice that the oxidation state of the carbon decreases with the number of hydrogens connected to it. On the contrary, the more oxygen bonds it has the higher the oxidation number. So, to reduce the carbon generally means adding more hydrogens to it replacing the bonds with more electronegative atoms:
Now, the carbonyl group contains a π bond, therefore, it is a center of unsaturated carbon atoms, therefore, we can add hydrogens to it converting the π bond into single bonds, much like we have seen in the hydrogenation of alkenes and alkynes:
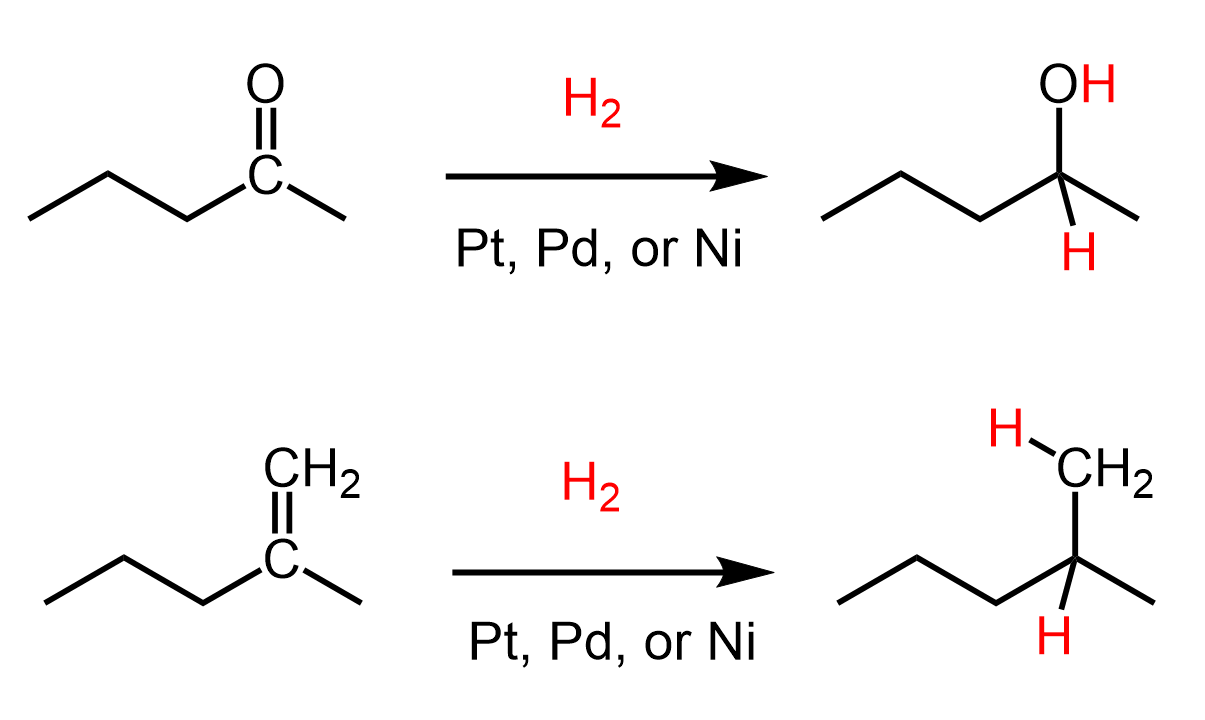
The catalytic hydrogenation, however, is not the most optimal method as it is a heterogenous reaction, involves the use of flammable hydrogen gas, may interfere with other functional groups, etc. It normally works with aldehydes and ketones, but more often, Lithium Aluminium Hydride (LiAlH4 or LAH) and Sodium Borohydride (NaBH4) are used for their reduction to alcohols.

Both LiAlH4 and NaBH4 are sources of hydride ion (H–), and unlike the catalytic hydrogenation, here we have an ionic mechanism where the hydride attacks the carbon of the carbonyl group. This addition is due to the polar nature of the C=O bond; the electronegative oxygen makes the carbon electron-deficient and becomes susceptible to a great number of important nucleophilic addition reactions that you are going to see in the upcoming chapters.
One type of these reactions is the reduction by hydride ion sources such as LiAlH4 and NaBH4. As a big picture, you can view any reduction of a carbonyl compound, as shown below:

The source of the hydride ion is the presence of a polar covalent bond between a metal and hydrogen. Because of higher electronegativity, the hydrogen bears higher electron density which eventually makes it react as a hydride ion:

For example, the Al-H bond in LiAlH4 is so polar that it has a nearly ionic character leaving the hydrogen as a hydride ion which is very reactive both as a base and a nucleophile:

The hydride ion reacts with the carbonyl group which, in turn, is also a polar covalent bond and the presence of the π bond makes the H– addition possible:

LiALH4 is one of the most powerful reducing agents efficiently working for any carbonyl and some other functional groups as well.
This high reactivity of the hydride ion in LiAlH4 makes it incompatible with protic solvents. For example, it reacts violently with water and therefore, LiAlH4 reductions are carried out in dry solvents such as anhydrous ether and THF.
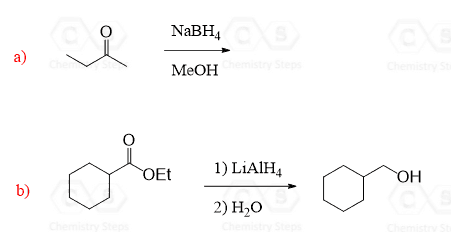
NaBH4, on the other hand, is not so reactive and can be used, for example, in a selective reduction of aldehydes and ketones in the presence of an ester:

Notice that LiAlH4 and NaBH4 reduce aldehydes and ketones to primary and secondary alcohols respectively. Esters, on the other hand, are converted to primary alcohols by LiAlH4.
LiAlH4 Reduction of Aldehydes and Ketones – The Mechanism
As mentioned earlier, both reagents function as a source of hydride (H−) which acts as a nucleophile attacking the carbon of the carbonyl C=O bond, and in the second step, the resulting alkoxide ion is protonated to form an alcohol.
There are, however, some differences depending on the reagent and to address those, let’s start with the mechanism of LiAlH4 Reduction:

The hydride addition to the carbonyl is also catalyzed by the lithium ion which serves as a Lewis acid by coordinating to the carbonyl oxygen. This decreases the electron density on the oxygen thus making the C=O bond more susceptible to a nucleophilic attack.
The resulting alkoxide salt can react with the AlH3 and convert it to another source of hydride. However, for simplicity, most often we show only one addition to the carbonyl followed by a protonation of the alkoxide with water or aqueous acidic solutions which gives the final product alcohol.
NaBH4 Reduction of Aldehydes and Ketones – The Mechanism
Sodium borohydride reduces aldehydes and ketones by a similar mechanism with some important differences that we need to mention.
First, NaBH4 is not so reactive and the reaction is usually carried out in protic solvents such as ethanol or methanol. The solvent has two functions here:
1) It serves as the source of a proton (H+) once the reduction is complete
2) The sodium ion is a weaker Lewis acid than the lithium ion and, in this case, the hydrogen bonding between the alcohol and the carbonyl group serves as a catalysis to activate the carbonyl group:

The Stereochemistry of LiAlH4 and NaBH4 Reduction
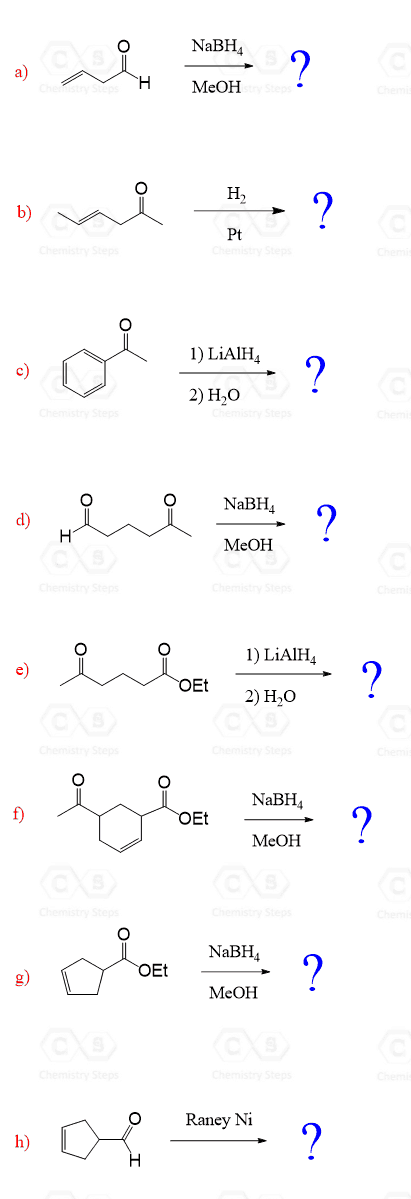
The reduction of unsymmetrical ketones with LiAlH4 or NaBH4 produces a pair of stereoisomers because the hydride ion can attack either face of the planar carbonyl group.

The same principle applies to the catalytic hydrogenation of the carbonyl group:

If no other chiral center is present, the product is a racemic mixture of enantiomers.
In the next article, we will discuss the reduction of esters and carboxylic acids as well.