From General to Organic Chemistry: What to Expect and How to Prepare
That gen chem class was tough, but you made it, or wait, it wasn’t that bad, and you don’t know why everyone was crying about… Anyway, you’ve got it – congratulations! Now, you’re entering a new chapter: Organic Chemistry.
So, what is it going to be like?
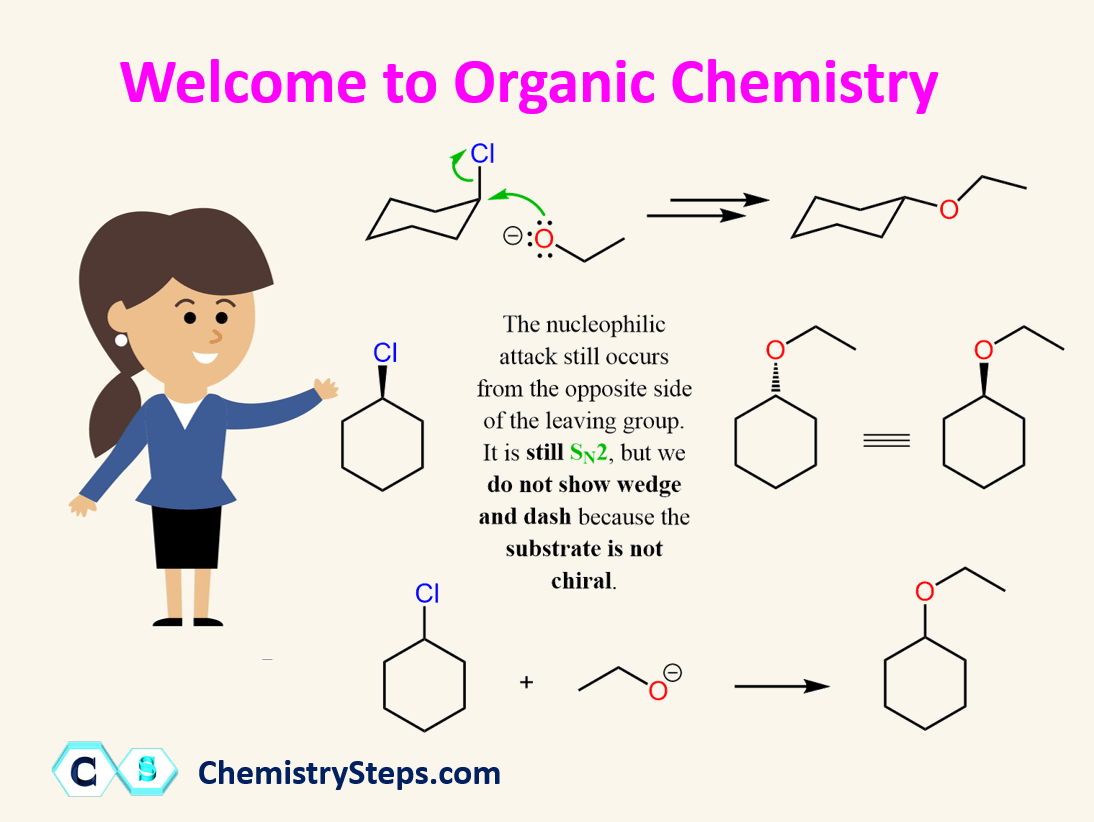
If you’ve heard rumors that this is the “hardest course in the premed path,” take a deep breath. While Organic Chemistry is certainly challenging, it’s challenging in a completely different way than General Chemistry.
Let’s walk through what to expect and the key concepts you must review before you begin.
Organic Chemistry is a different type of Chemistry
Here’s the good news:
All those math-heavy, calculation-based problems in General Chemistry, like thermodynamics, equilibrium, and rate laws? You’ll barely see them again.
Organic Chemistry is much more conceptual. It’s like learning a new language:
- Less calculator, more visual reasoning
- Less formulaic solving, more curved arrows
- Less plug-and-chug, more structural understanding
You’ll shift from solving numeric problems to understanding how and why molecules react. It’s not about memorizing equations – it’s about learning how atoms and molecules behave.
Key Advice: Don’t Just Think – Draw ✏️
You won’t believe how many times I have heard from a student, “I can’t do this”, yet when I asked them to take the pencil and start from somewhere, it appeared that they got half of the problem solved. For example, you need to draw the enantiomer of a molecule (think of it as a different variation of the molecule), and you are stuck, not knowing what to do. Take a pencil and just draw something similar to the original molecule. Yes, it is that simple – you need to draw out structures, and that is the only way of moving forward in organic chemistry.
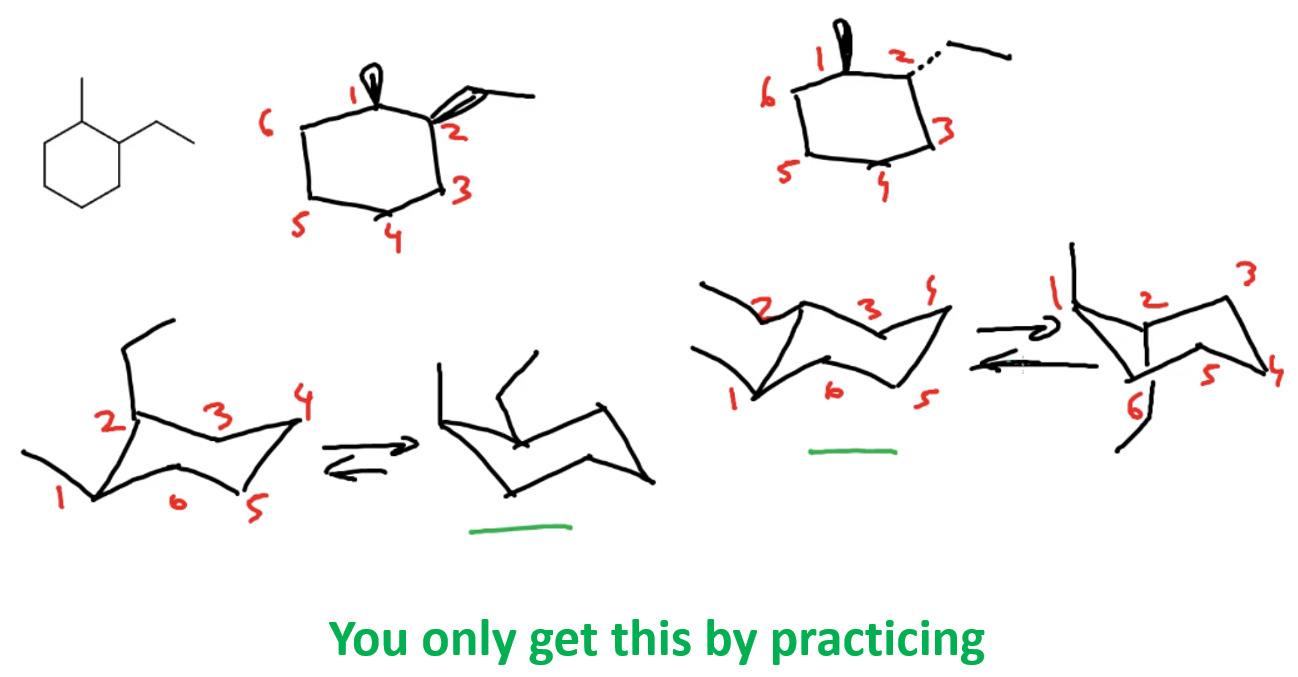
They say think before you do, but strangely enough, often times, you do before thinking in organic chemistry (don’t do this in the lab though). The reason is that once you have something drawn out, you can work on it, you can analyze, compare, ask your friend, instructor, or whoever. Just like in math, you don’t read the problem once and start solving it all in your head. You write down something and go from there.
✅ So, Pro tip: It’s often better to start drawing, even if you’re not sure what you’re doing.
Drawing structures, mechanisms, or resonance forms gives you something to work with and gets you going. It is okay if it is wrong – nobody cares or judges you, until it’s test time, so use that window to master visualizing and drawing organic molecules.
Practice, Practice, and Practice More
 You wouldn’t like someone teaching you how to swim or bike by inviting you to a café, would you? Sure, you need some chit-chat on what to do and what not, but the only thing that matters is having the bike so you can ride, fail, repeat 100 times till you get going. The same is here, do not just listen to your professor and be like, “oh yeah, I got this” – you don’t, and before you know, you have a test the next day, and you are crying from cramming all the things that you did not do yourself. So, attend the class, take notes, read your textbook, watch YouTube or whatever works for you best, but close/pause it, and do it yourself!
You wouldn’t like someone teaching you how to swim or bike by inviting you to a café, would you? Sure, you need some chit-chat on what to do and what not, but the only thing that matters is having the bike so you can ride, fail, repeat 100 times till you get going. The same is here, do not just listen to your professor and be like, “oh yeah, I got this” – you don’t, and before you know, you have a test the next day, and you are crying from cramming all the things that you did not do yourself. So, attend the class, take notes, read your textbook, watch YouTube or whatever works for you best, but close/pause it, and do it yourself!
🔑 And now, more practical – What You’re Expected to Know on Day One
You are not going to need stoichiometry, ICE table, the math of thermodynamics and electrochemistry. They are not about the structure of molecules.
Take a couple of minutes to guess – what do you need from general chemistry that is important to understand molecular structures?
……….
You guessed it right, this is the list of main topics you need for organic chemistry:
Lewis Structures, Hybridization, and VSEPR Theory
What Are Lewis Structures and Why Do They Matter in Organic Chemistry?
A Lewis structure is a diagram that shows how atoms are connected in a molecule, what types of bonds are there, which atom(s) have a lone pair of electrons, there are any formal charges?

You cannot draw a correct chemical structure without understanding Lewis structures, and we have already mentioned that organic chemistry is all about drawing molecular structures.
So, review Lewis structures to locate the sigma and pi bonds, lone pairs, and formal charges, and determine the electronic and molecular geometries of molecules using the VSEPR theory.
The good news about hybridization and VSEPR theories is that you never go beyond the sp3/tetrahedral geometry because organic chemistry is all about carbon, which cannot have more than four bonds.

As you pass the initial part of the semester, you are going to need all of this to understand what electron-rich (nucleophilic) or electron-poor (electrophilic) molecules are, and this is very important in understanding nucleophilic substitution and elimination reactions.
Bond-Line Structures – We Skip Carbons In Organic Chemistry
You just said Ochem is all about carbon!? Yes, it is, and this is what I mean by “We skip carbon in organic chemistry”. Organic molecules are often a lot larger than what you saw in general chemistry, and because they all consist of carbons, it is often convenient not to show the carbon atoms. What we do is simply draw a zig-zag line, which presumes a carbon at every angle.

This is called bond-line and zig-zag structures, and it is something new compared to general chemistry. It takes time to get used to it, so if you have time, go over it to have a better foundation in understanding organic structures.
🔊 Stereochemistry
Stereochemistry is about the 3D geometry of the molecule, and you are not expected to know it before starting your organic chemistry class. However, it would be very beneficial to first of all realize/recall that molecules are not flat as we show them in gen chem classes.

For example, you know that the simple methane (CH4) is tetrahedral, and we show two of it hydrogen atoms with what is called wedge and dash notations. The carbon is sp3-hybridized and has a tetrahedral geometry. To show this geometry, two of the hydrogens are drawn in the plane of the paper, and the other two are pointing toward and away from the viewer. This is where the wedge and dash notation is used: the atoms pointing towards us (in front of the plane) are shown with a wedge line, and the one pointing away (behind the plane) from us is represented with a dashed line:
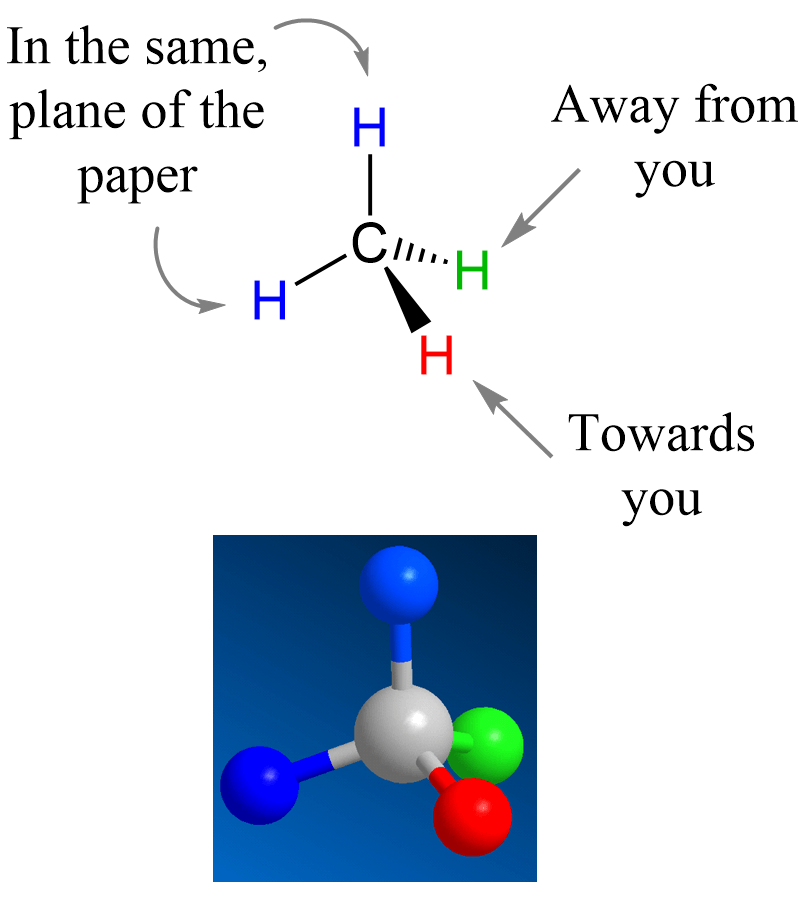
Most often, after the introductory chapters, organic molecules are going to be shown in bond-line representations where the carbon and hydrogen atoms are not shown except when connected to heteroatoms. So, for methane, we can also show it as:
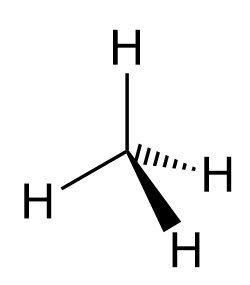
Let’s now look at the wedge and dash notation for the hydrogens in ethane. We have two sp3 carbons connected, and therefore, on each is going to be a wedge and a dash hydrogen:
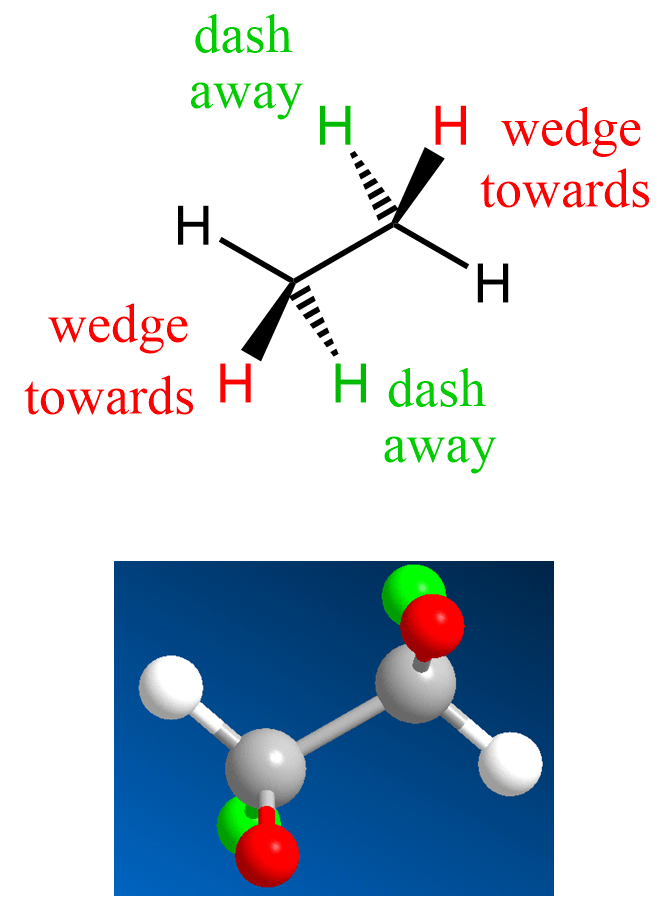
Notice that, as drawn, the other two hydrogens are in the same plane as the carbon atoms. Keep in mind, however, that there is a free rotation about single bonds, and therefore, these hydrogens are not fixed in the same plane. It is rather the most convenient way of drawing them.
This, essentially, is what stereochemistry is about. It is not that simple, of course, different spatial arrangements of atoms give different 3D geometries to the molecule, thus different types of stereoisomers, such as enantiomers and diastereomers, arise. Again, you do not have to worry about it now, but make sure you understand VSEPR theory, molecular geometry, and what wedge and dash notations indicate.
Acids and Bases
Organic acids and bases are going to be the starting point of dealing with chemical reactions in organic chemistry. While the first part of the class is going to focus on understanding and drawing organic molecules, here, you will also start applying your knowledge of acid-base chemistry from general chemistry to diving into the reactions in organic chemistry.

Make you know what pKa is, what it tells about the acid strength of the molecule, to further understand the driving force of many organic reactions. Notice that, unlike what you did in general chemistry, you are also going to use curved arrows. These are the foundations of understanding reaction mechanisms.
Every curved arrow has a head and a tail for showing the flow of electrons from a high electron density to a low electron density center. The arrow must start from the middle of a lone pair or a covalent bond.

For example:

In this reaction, the electrons move from the Cl to the carbon, and as a result, a new bond is formed.
Once again, no need to worry about organic chemistry mechanisms for now, simply go over this article, and remember that curved arrows show movements of electrons. After all, chemical reactions are a result of electrons moving from one atom to another, thus making and breaking bonds.
Polarity and Bond Types
Unlike general chemistry, where we learn about acids, bases, salts, electrolytes, etc., in organic chemistry, we mostly work with covalent molecules. So, review the principles of covalent bonding, understand what makes a polar or nonpolar covalent bond, and which atoms get a partial positive or a partial negative charge.

We have some articles in the general chemistry section, as well as those here, in the organic chemistry section, that would help you review these concepts.
Understanding electronegativity and bond polarity becomes very important towards the end of the organic chemistry 1 class because this is when you start talking about nucleophilic substitution and elimination reactions. These are normally carried out on alkyl halides, which contain a carbon-halogen (C-X) polar bond. Once again, most organic reactions occur because of an imbalance in the electron distribution among some atoms. This is a result of having polar covalent bonds because they create partially charged reactive sites.
Summarizing What to Expect in Organic Chemistry
Organic chemistry is not a continuation of general chemistry – it’s a whole new mindset. You’ll be trading your calculator for colored pens and curved arrows. The focus shifts from solving equations to understanding how molecules behave, how electrons move, and how structure influences reactivity.
Here’s what you should take away:
- ✅ You don’t need to worry about all the heavy math from Gen Chem 2. You can leave behind most of the Gen Chem math, like ICE tables and thermodynamics.
- ✅ What you do need is a solid understanding of Lewis structures, hybridization, VSEPR, acid-base concepts, and bond polarity.
- ✅ Drawing is everything. Mechanisms, resonance, stereochemistry – none of it makes sense unless you’re drawing and thinking visually.
- ✅ Don’t just stare at problems. Draw something – even if it’s wrong. That’s how you learn and improve.
- ✅Don’t wait until the night before the test to suddenly “get” organic chemistry. It’s not the kind of subject you can cram. You need to practice regularly, just like you can’t learn to ride a bike by watching videos or reading about it, you can’t master organic reactions without doing problems over and over.
The most important part in all of this is that you are thinking ahead, and that is why you are reading this article. This means you are ahead of many of your classmates, and you are set to succeed in Organic Chemistry!
You are going to visit Chemistry Steps many times during the semester because of the resources, such as hundreds of articles, practice problems, and quizzes, that we have to offer. Use the code “JoinChemSteps” to get a 20% off on our Monthly and Semester memberships here.
Thank you for stopping by, and I hope this was helpful.
Let me know in the comments if you have any questions. Have you started Organic Chemistry yet? Do you need some guidance? Do you feel lost?

Gevorg Sargsyan – Dr. S.
The founder and author of Chemistry Steps
Bachelor’s degree in Chemistry – YSU, Armenia, 2001-2005
Ph.D. in Organic Chemistry – UWYO, USA, 2008-2013
PostDoc, Supramolecular and Biological Chemistry, UNIGE, Switzerland, 2014-2015
Chemistry Instructor – STC, USA, 2015-2020
