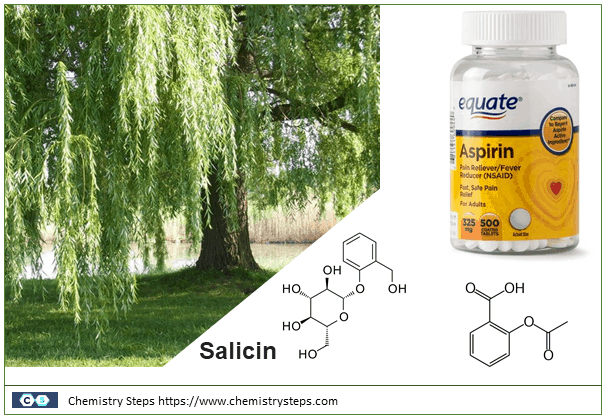
Salicin is found in willow trees and has been used ever since the 18th century for its analgesic and anti-inflammatory properties. It is composed of glucose and salicyl alcohol linked by an ether bridge which breaks and the alcohol is eventually metabolized as salicylic acid which is the active component of aspirin (acetylsalicylic acid).
As for studying, check this comprehensive problem (#5) on Aspirin that covers stoichiometry including the limiting reactant and percent yield of the reaction.
More chemistry material in the Student Help section.

Pretty! This has been an incredibly wonderful post.
Thank you for supplying these details.