In the IUPAC nomenclature, nitriles can be named by systematic and common nomenclature.
Systematic Nomenclature of Nitriles
In the systematic nomenclature, the suffix “nitrile” is simply added to the name of the parent alkane that contains the CN group. The parent chain is numbered by putting the CN at carbon 1. This number, however, is omitted from the name since it presumes that the CN being the highest priority must be the C1:

Naming Nitriles on a Ring
When the -CN group is on a ring and it is the highest priority, we add the suffix “carbonitrile”:

Notice that, unlike the linear nitriles, the CN carbon here is not counted in the parent chain and the position of the CN group is not indicated when there are no other groups on the ring. However, if substituents are presnet, the numbering starts from the carbon connected to the CN group and goes in the direction that minimizes the numbering of the substituents:
Common Names of Nitriles
Nitriles are derivatives of carboxylic acids and if you have already read about the nomenclature of carboxylic acids, you are familiar with suffixes -ic acid, -oic acid. So, in the common nomenclature, all you need to so is replace the -ic acid, -oic acid, or ending with the suffix “onitrile”:

If a higher priority group is present, then the nitrile is considered as a substituent indicated with the prefix “cyano”. It is placed alphabetically just like any other substituent:
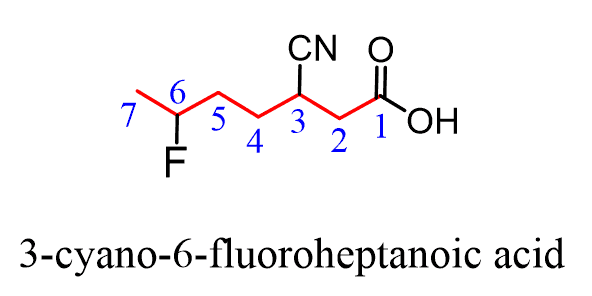
Below are some practice examples for naming different derivatives of carboxylic acids including nitriles.


Nitriles do not feature much in UK ‘A’ Level Chemistry syllabi, but I found the above interesting. Thank you for putting it on the web.
Yours sincerely
Angus Phaure