The nomenclature of alkyl halides follows the same IUPAC rules that we discussed for naming alkane. The only difference here is that one (or more) of the substituents is a halogen, and its naming is a little modified.
Let’s first recall the nomenclature rules by naming the following alkane:
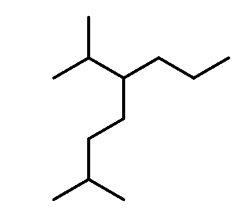
Step 1. Find the parent chain. The longest possible chain here consists of eight carbons, so the parent chain is octane.
If there are two chains of equal length, then you need to choose the one with the greater number of substituents. Check the nomenclature of alkanes for more details.
Step 2. Find the substituents. In this case, we have a methyl and an isopropyl group.
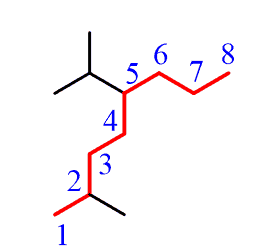
Step 3. Number the parent chain, giving the lowest possible numbers to the substituents:
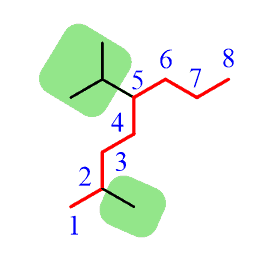
Step 4. Put the parent chain and substituents together by placing the substituents in alphabetical order!
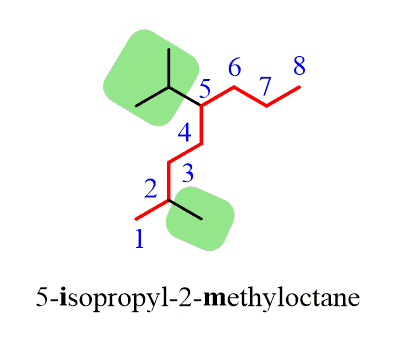
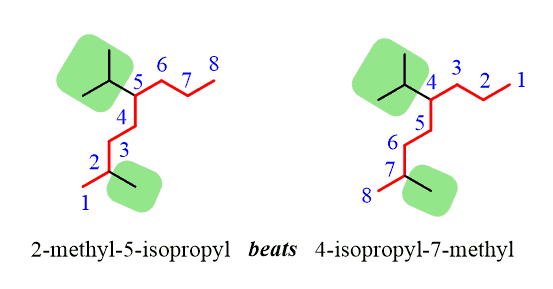
Notice that even though the isopropyl group is at position 5, it is still placed before the methyl group at position 2 since the substituents are placed alphabetically.
It is worth mentioning that it is only the “iso” prefix that is considered for alphabetical priority. All the other common groups shown below are based on the first letter of the carbon chain and not the prefix:

Naming Alkyl Halides
The halogen in alkyl halides is treated just like any alkyl substituent, meaning it has no priority over the carbon atoms. The parent chain is still numbered in a way to give the lowest possible number(s) for the substituents.
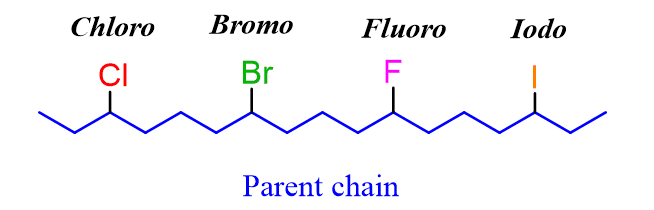
The only difference in naming alkyl halides is the change of the suffix “ine” to “o”. For example, 2-bromo, 4-chloro, 3-iodo, 5-fluoro:
Let’s name the following alkyl halide:
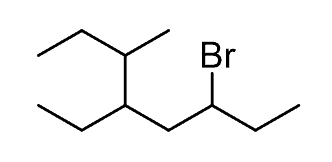
Step 1. Find the parent chain. The longest possible chain consists of eight carbons, so the parent chain is octane:

Step 2. Find the substituents. Here, we have three substituents – two alkyl groups and a halide:
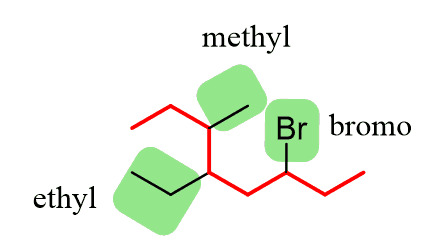
Step 3. Number the parent chain, giving the lowest possible numbers to the substituents. If there is a tie for the first locant, compare the second and then the third locants.

Step 4. Put the parent chain and substituents together by placing the substituents in alphabetical order:
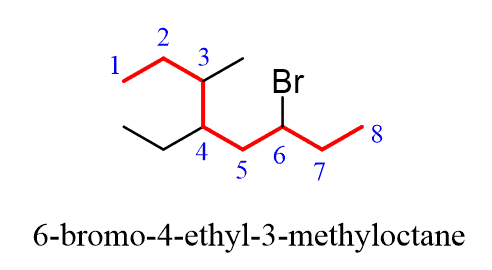
Notice again that in the final name, the groups are placed in alphabetical order. Even though Br is in position 6, it is still placed before the other substituents.
Naming Alkyl Halides with Chiral Centers
When a stereogenic center is present in a compound, its absolute configuration needs to be indicated according to the Cahn-Ingold-Prelog rules.
For example, what is the IUPAC name of the following alkyl halide?
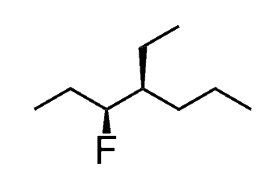
The chiral center does not change any of the rules we discussed above! Therefore, we first find the parent chain followed by substituents, number the parent chain by giving the lowest possible locants, and finally put everything together in alphabetical order:

The only difference with chiral molecules is that you need to determine the R and S configurations and place them with the corresponding locant at the beginning of the name.
Check out this 65-question, Multiple-Choice Quiz with a 3-hour Video Solution covering Nucleophilic Substitution and Elimination Reactions:


