We have learned that activating groups are ortho, para directors, and the meta product for these benzene derivatives is obtained only in negligible quantities. To obtain a meta-substitution product, a deactivating group such as a nitro or a carbonyl group is needed.
Any Activating group directs the electrophile to the ortho and para positions.
Any deactivating group directs the electrophile to the meta position.

Sometimes, though, you may be asked to synthesize a benzene ring that has activating groups in meta positions. For example, how would you prepare 1,3,5-tribromobenzene?
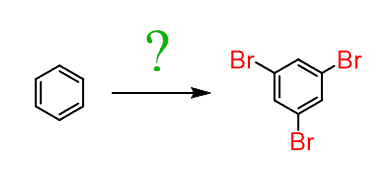
1,3,5-tribromobenzene cannot be prepared by the direct EAS halogenation since halogens are ortho-, para-directors and once the first bromine is installed on the ring, it will direct the others to ortho and para positions:

This problem can be overcome by a useful feature of an arenediazonium salt, which is that it can be removed from the ring once the desired transformation is achieved. And this is the reaction with hypophosphorous acid. As a reference, here are the main reactions of arenedizaonium salts:

So, the strategy is to prepare aniline, brominate it at the ortho and para positions, and finally remove the NH2 group by converting it into a diazonium salt:
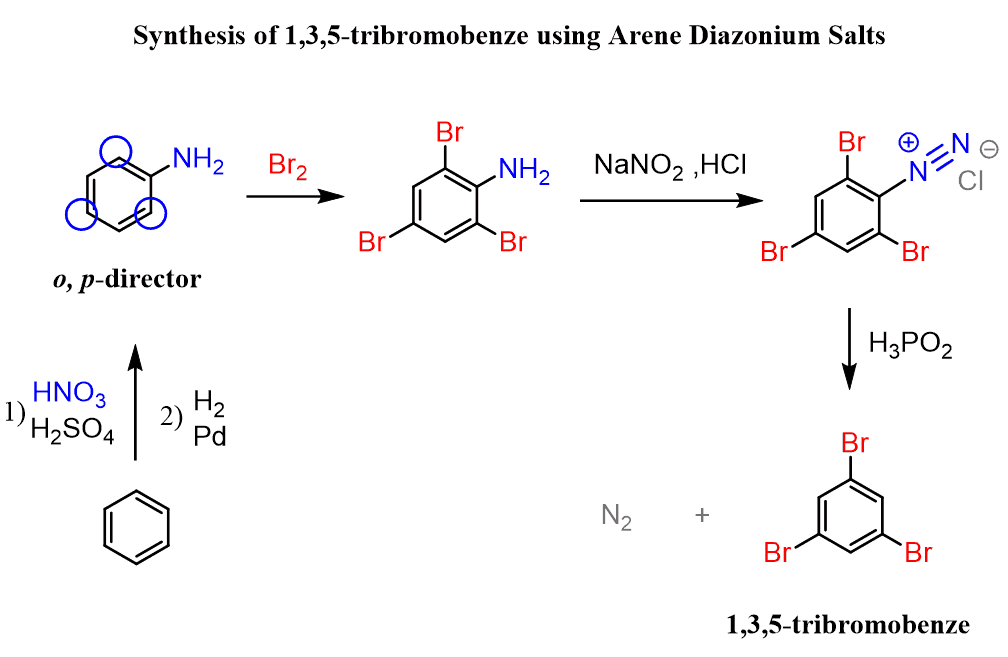
Notice that the bromination of aniline did not require a catalyst because it is a very strong activator, and the reaction is impossible to stop at monobromination even in these conditions.
Let’s consider another example: How can we synthesize resorcinol (benzene-1,3-diol) from benzene?
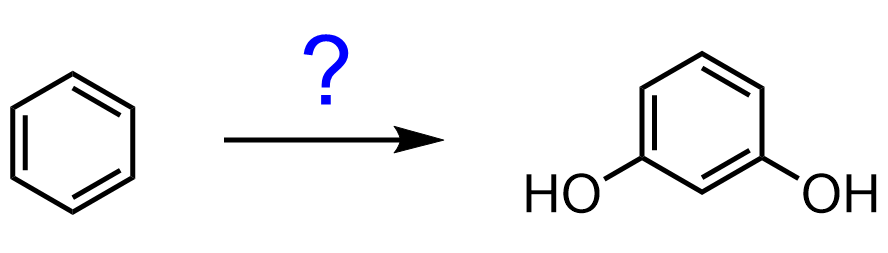
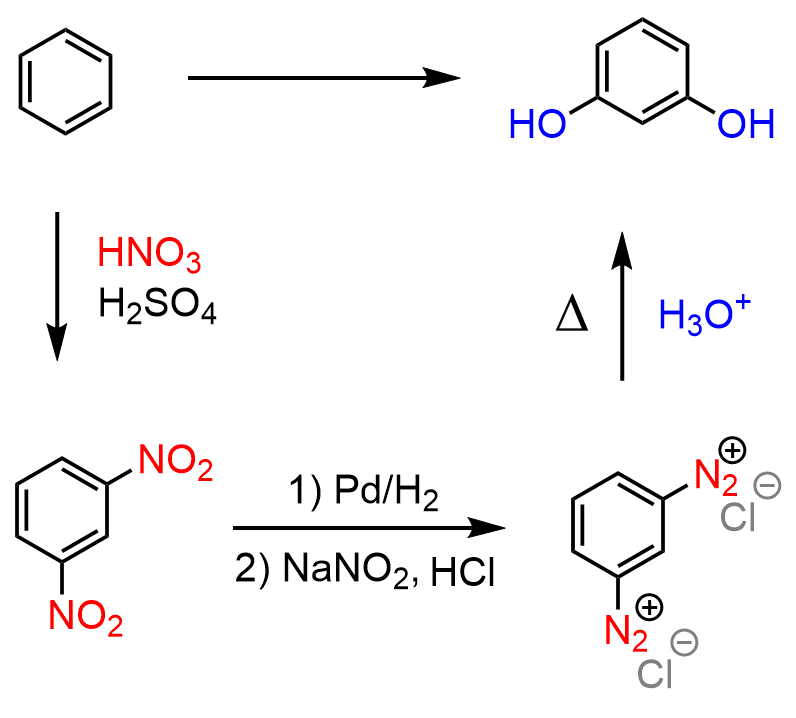
Two groups at meta positions indicate a nitro intermediate, which is easily converted to a diazonium ion. Once we have the diazonium salt, we can hydrolyze it to OH:
Sometimes, a meta substitution can occur as a nondesired product, although if the reaction is known and the yield is suitable, it can be a synthetic strategy too.
For example, even though the amino group activates the benzene ring, and some electrophilic aromatic substitution reactions of aniline are faster compared to those with benzene, nitration of aniline does not work as expected. We know that activating groups are ortho, para directors, so expecting ortho, para nitration of aniline might seem a reasonable prediction.
However, it has been shown that this reaction either leads to a mixture of undesirable side products, or, what is interesting, meta-nitroaniline is obtained:
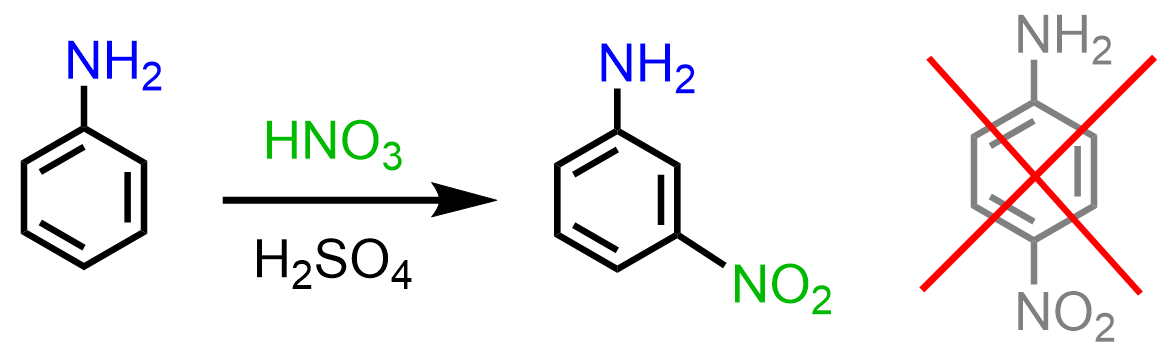
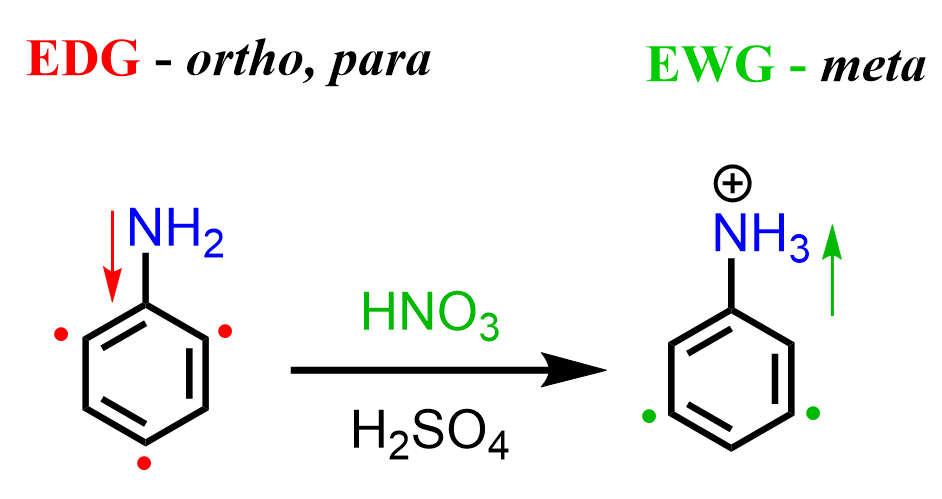
The formation of the unexpected meta product is again due to the basicity of the amino group. While in Friedel Crafts reactions, it acts as a Lewis base, here, we have a regular Bronsted acid-base reaction with HNO3 and H2SO4. Once the amino group is protonated, a strongly deactivating -NH3+ group is formed, which now undergoes a meta EAS:
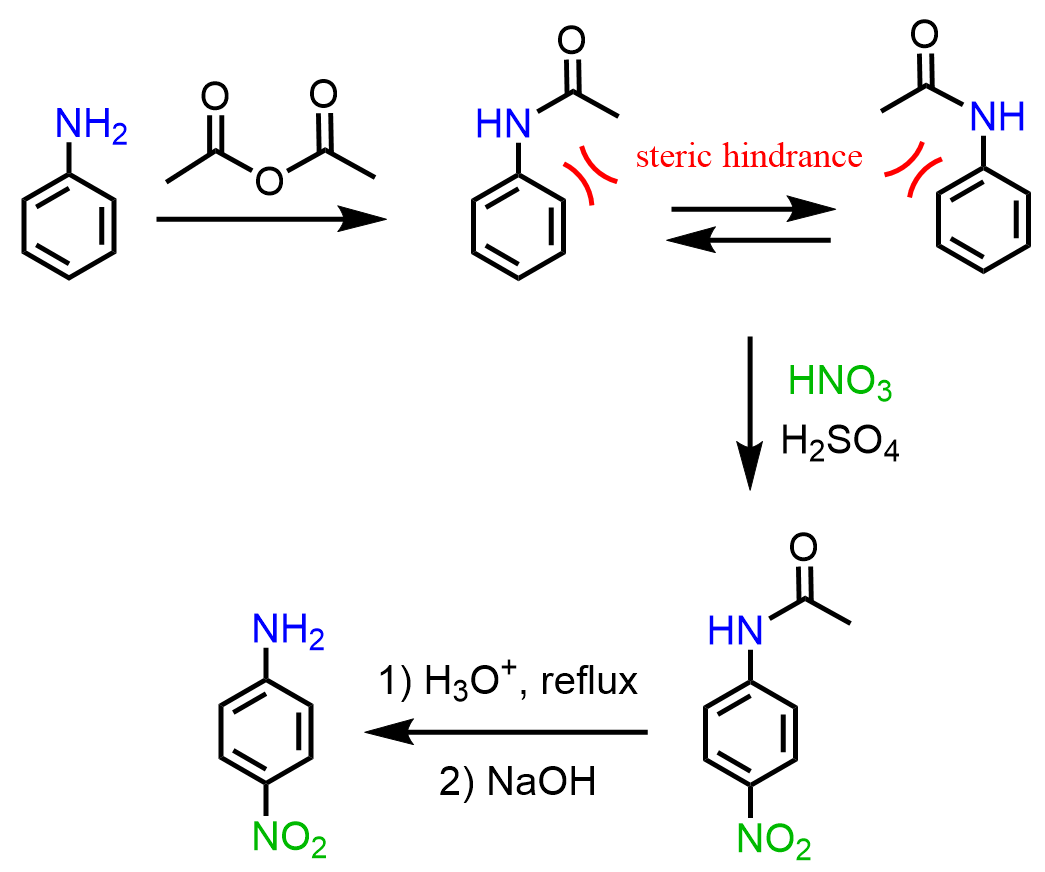
If the target product is the para product, the strategy is to convert the amino group to an amide and hydrolyze it back to amine once the nitration is complete.
It is worth mentioning that the Friedel-Crafts reactions themselves are very slow, and meta alkylations or acylations do not work once the nitrogen binds to the Lewis acid catalyst and thus becomes a deactivator:
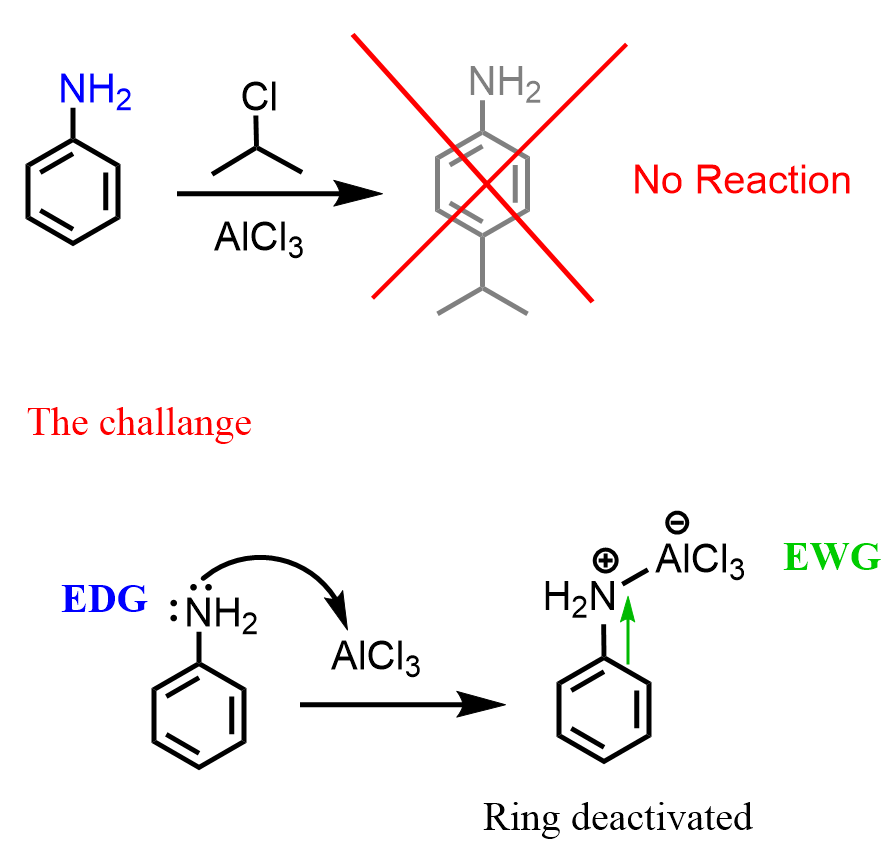
This is true for doing Friedel-Crafts on any deactivated benzene derivative – these reactions do not work.
Like for the nitration, if the target product were the para isomer, the strategy of acylating the amino group would be used. The difference between the amino and amide groups is that in the amide, the lone pair of the nitrogen is conjugated with the carbonyl group and is less available to be in resonance with the electrons of the ring:

Synthesis of Disubstituted Benzene Rings
The fact that Friedel-Crafts reactions do not work for deactivated rings must be considered for the planning of disubstituted benzene rings. For example, let’s consider another target product of two activating groups in meta orientation:
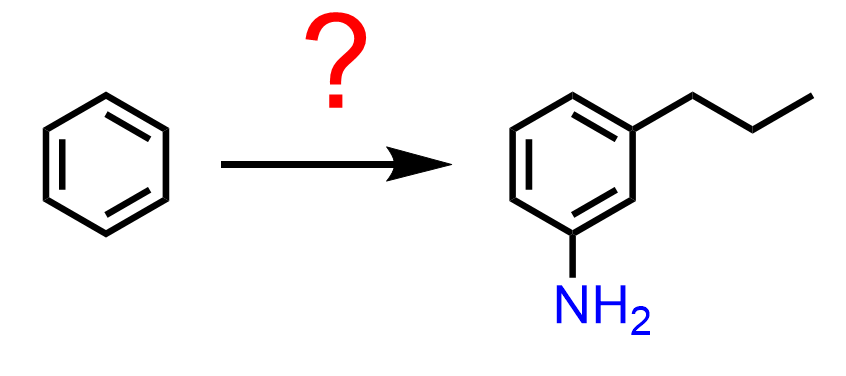
The amino group is usually prepped by the reduction of the nitro group, and, in addition, nitro is a meta director, which leads to the conclusion that the precursor of 3-propylaniline is 1-nitro-3-propylbenzene:
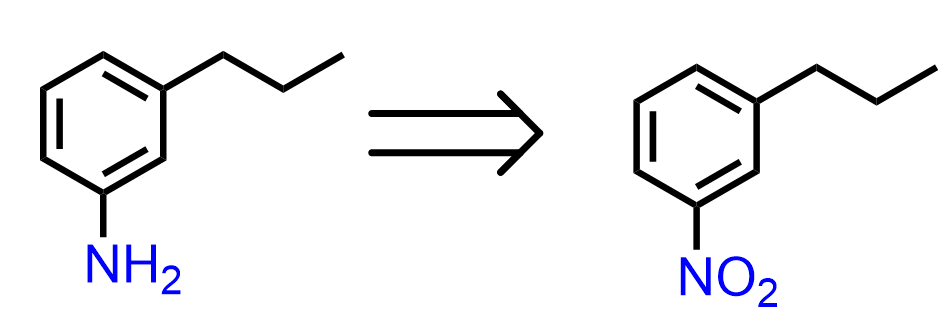
So, the next question is how do we synthesize the 1-nitro-3-propylbenzene? Nitrobenzene is a meta director, so can it be prepared by a Friedel-Crafts alkylation of nitrobenzene?
As we have mentioned earlier in the article, this reaction does not work because Friedel-Crafts do not work on deactivated rings, and in addition, even if the reaction worked, there would’ve been a rearrangement of the primary alkyl halide:
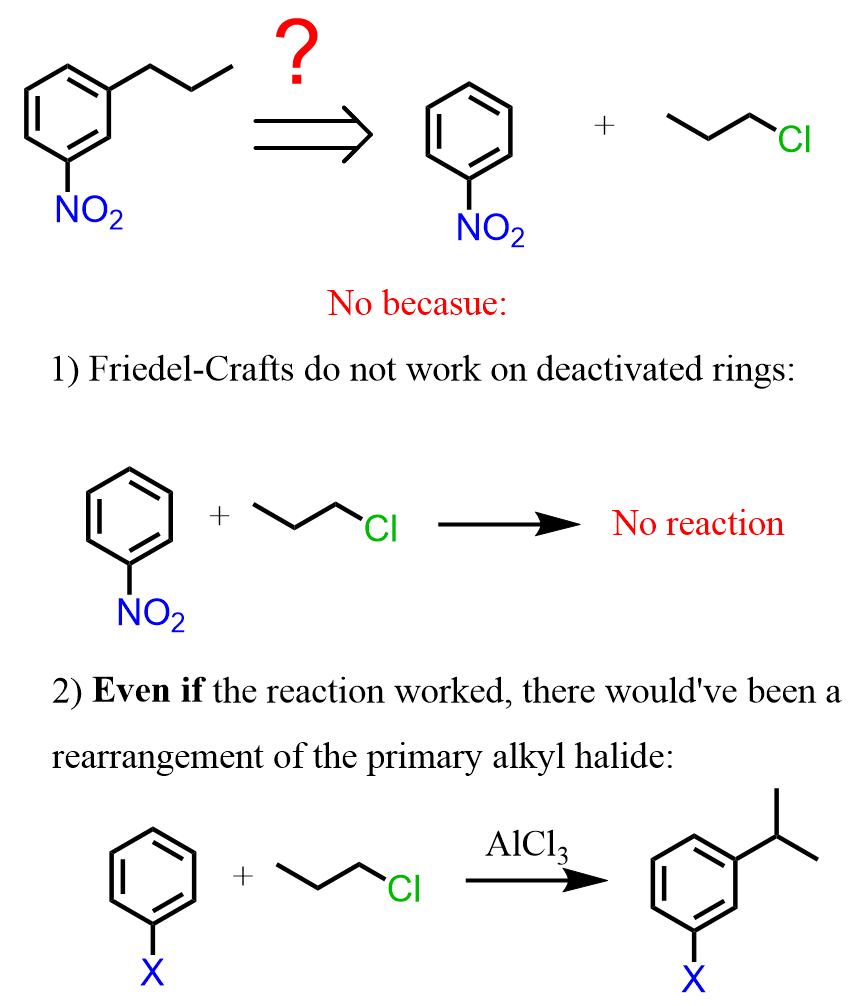
Therefore, the strategy is to do the Friedel-Crafts first, and then nitrate the ring. The question is, how do we add the nitro group in the meta position if the propyl group is an activator and an ortho, para director?
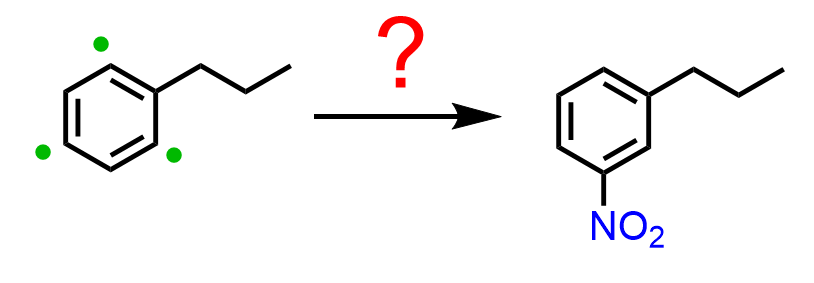
In these cases, Friedel-Crafts acylation followed by a reduction of the carbonyl is used. So, first, we prepare the aryl ketone via Friedel-Crafts acylation of benzene, and the reactive side is now the meta position, which allows us to add the nitro group. The final product with two activators in meta orientation is achieved by reducing the nitro and carbonyl groups:
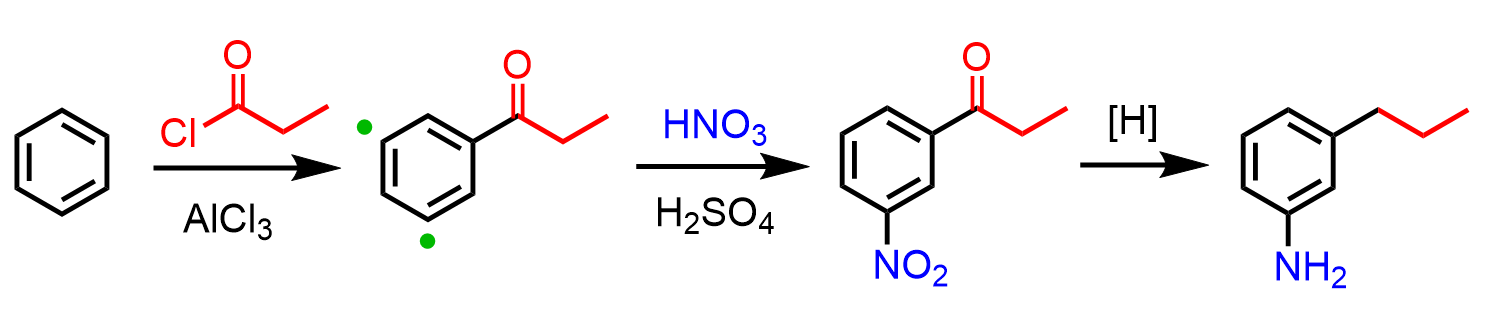
These were some limitations of EAS reactions and strategies to overcome them. If there are other common encounters, let me know in the comments, and we can discuss them further.
Check Also
- Electrophilic Aromatic Substitution – The Mechanism
- The Halogenation of Benzene
- The Nitration of Benzene
- The Sulfonation of Benzene
- Friedel-Crafts Alkylation with Practice Problems
- Friedel-Crafts Acylation with Practice Problems
- Vilsmeier-Haack Reaction
- The Alkylation of Benzene by Acylation-Reduction
- Ortho, Para, Meta in EAS with Practice Problems
- Ortho, Para, and Meta in Disubstituted Benzenes
- Why Are Halogens Ortho-, Para– Directors yet Deactivators?
- Is Phenyl an Ortho/Para or Meta Director?
- Limitations of Electrophilic Aromatic Substitution Reactions
- Orientation in Benzene Rings With More Than One Substituent
- Synthesis of Aromatic Compounds From Benzene
- Arenediazonium Salts in Electrophilic Aromatic Substitution
- Reactions at the Benzylic Position
- Benzylic Bromination
- Nucleophilic Aromatic Substitution
- Nucleophilic Aromatic Substitution Practice Problems
- Reactions of Phenols
- Reactions of Aniline
- Meta Substitution on Activated Aromatic Ring
- Electrophilic Aromatic Substitution Practice Problems
- Aromatic Compounds Quiz
- Reactions Map of Aromatic Compounds
