In the previous post, we talked about a simple strategy you can use for changing the position of a double bond.
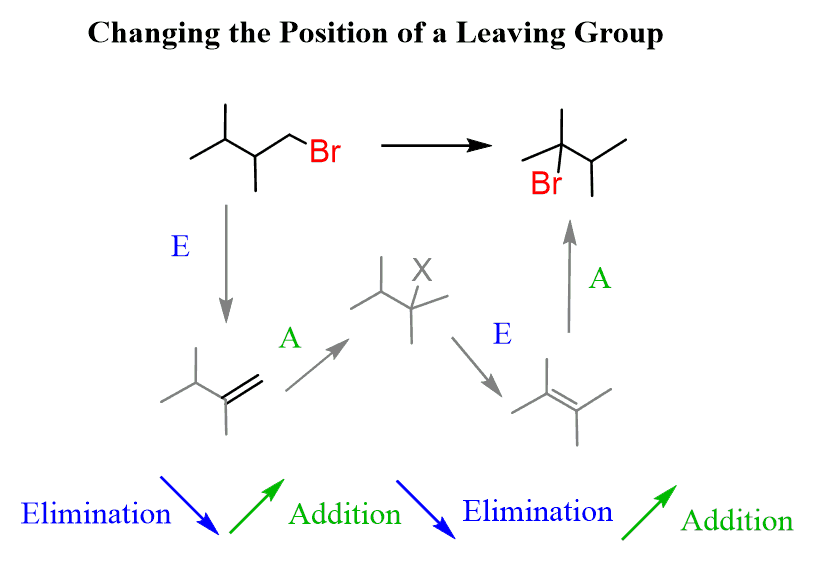
A similar tool-set, commonly used in organic synthesis, is the change of the position for a leaving group:

Here as well, we need to identify the direction where the leaving group is moving and apply the regioselective elimination based on the Zaitsev and Hofmann rules followed by regioselective addition to alkenes (Markovnikov’s rule).
Remember, you can selectively add a halogen to the most and least substituted positions of a double bond:
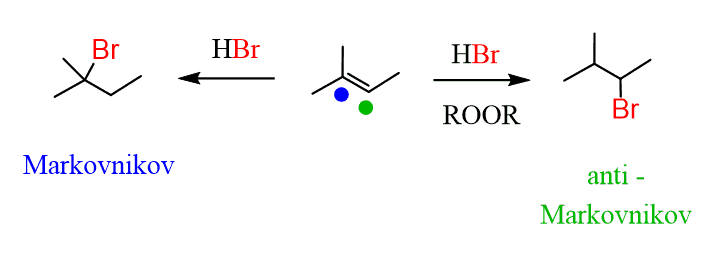
And you can also selectively introduce a double bond by E2 reactions using a non-hindered (Zaitsev) and a bulky (Hofmann) base:
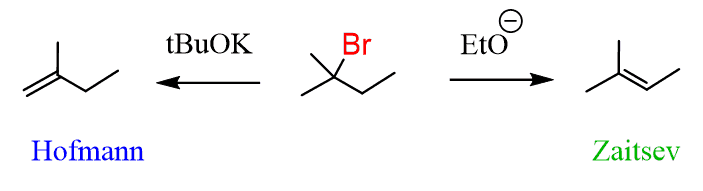
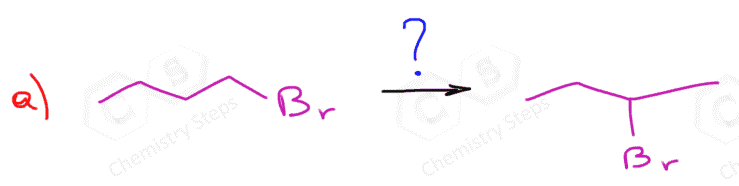
So, let’s follow these steps to solve our problem:
- Indicate the direction where the leaving group is moving:
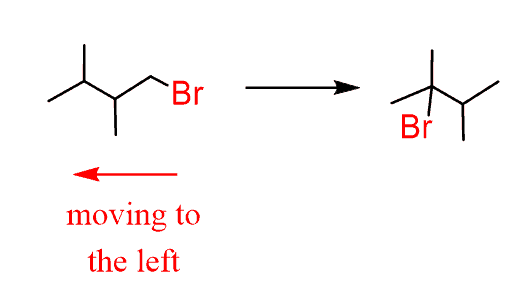
- Introduce a double bond to the left of Br. In this case, it doesn’t matter what base we use for the regioselectivity (hindered on unhindered) but we can use a bulky base to enforce elimination over substitution.
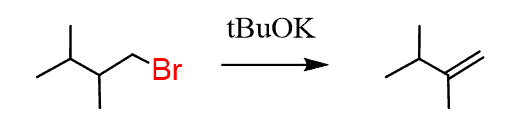
- Still moving to the left, so a Markovnikov addition is needed:
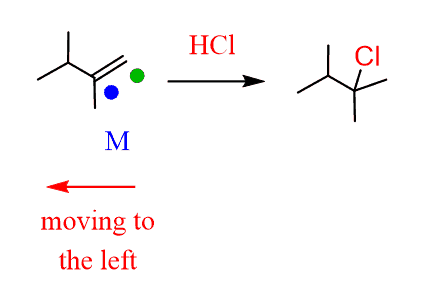
- Another elimination to the left, and this time we must use a small base to obtain the Zaitsev product:

- We need one more addition to the asymmetrical double bond:
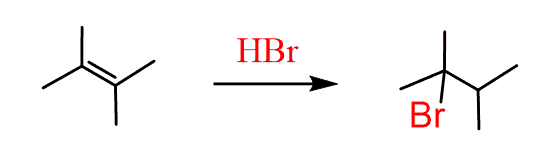
So, to summarize, for changing the position of a leaving group, you need to repeatedly apply regioselective addition and elimination reactions to the direction where the leaving group had moved:

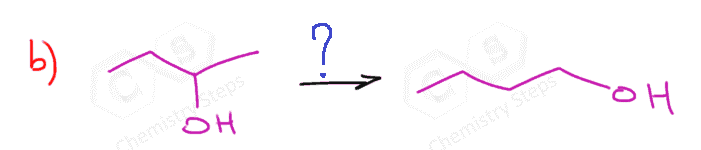
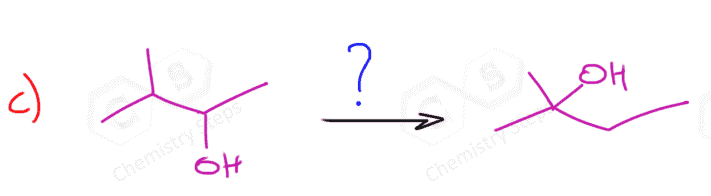
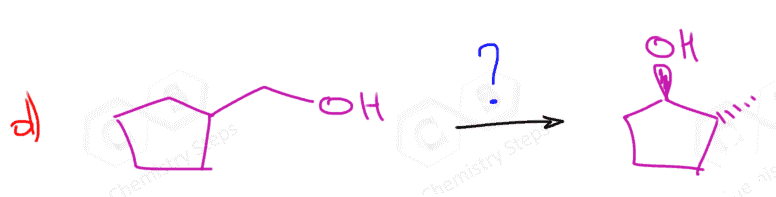





Thank you!! This is incredibly helpful.