At the beginning of this chapter, we learned that alkenes are electron-rich species and, therefore, undergo a variety of electrophilic addition reactions. To convert them to epoxides, we want to add an oxygen atom to a double bond. This oxygen needs to be electron-deficient (electrophilic), which is not an obvious task because of oxygen’s great electronegativity.
Fortunately, it has been found that the oxygen in peroxycarboxylic is electron-deficient and when mixed with alkenes, an addition to the double bond occurs giving a cyclic structure called an epoxide:

Representative examples of peroxycarboxylic acids are MCPBA (meta-chloroperoxy benzoic acid) or Peroxyacetic acid which we have seen in the ani-dihadroxylation of alkenes.
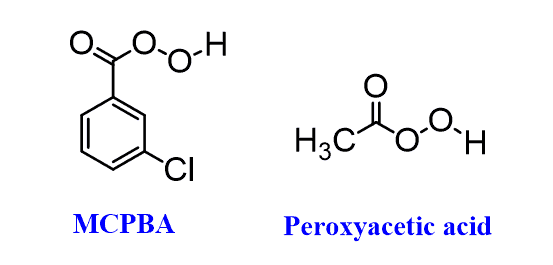
The reason for the popularity of mCPBA is that unlike many peroxycarboxylic acids needed to be prepared right before the reaction by mixing a carboxylic acid and hydrogen peroxide, it is a quite stable crystalline solid and can be purchased commercially.
This does not, however, exclude it from the characteristic of the peroxides danger of detonation if not handled properly.
Now, the fact that peroxides have low stability indicates that they are very reactive, and this is explained by the presence of the weak O-O bond. This, in the presence of a weak π bond, is a good combination to drive the epoxidation reaction forward.
The Mechanism of Epoxidation of Alkenes
The oxygen in the peroxide is electron-deficient and is attacked by the p electrons of the π bond. At the same time, the lone pair of the oxygen also acts as a nucleophile attacking one of the carbon atoms of the alkene.

The weakness of the O-O bond makes the bond cleavage easier thus acting as a driving force for the epoxidation.
This mechanism is similar to the formation of halonium ion and oxymercuration which we have also discussed in the other addition reactions of alkenes.
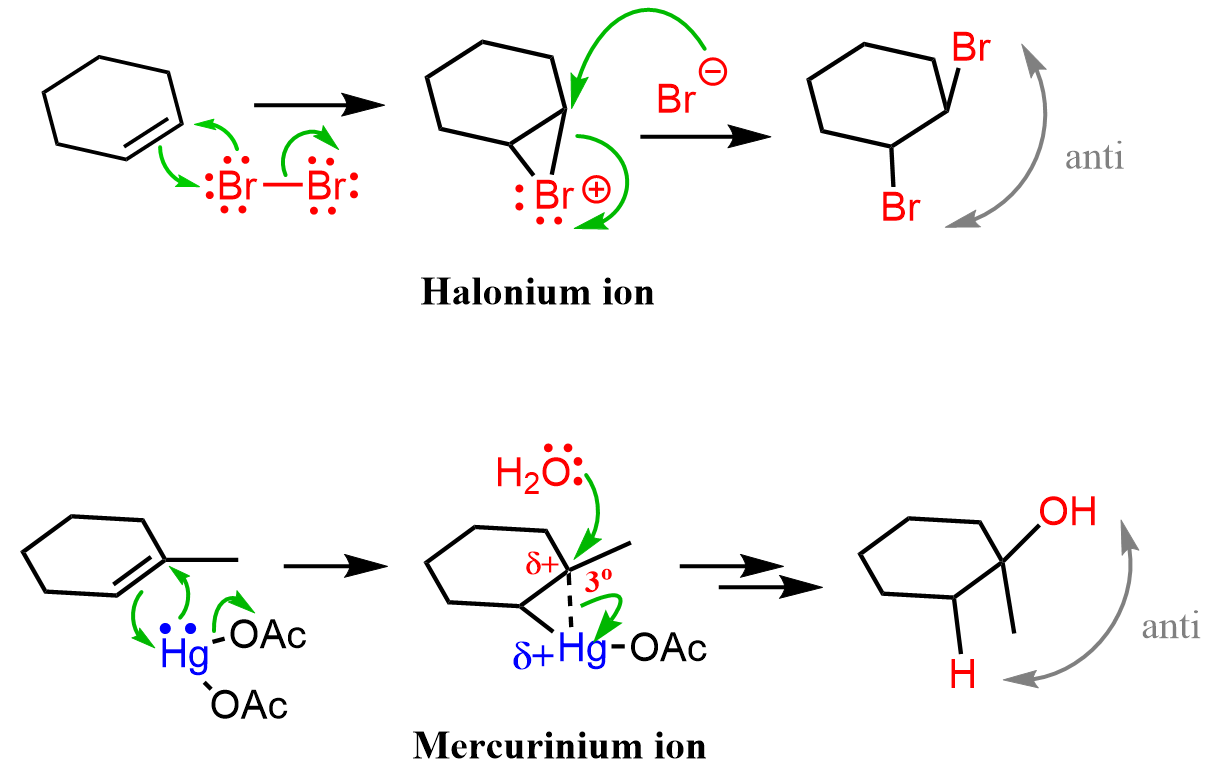
An alternative, short, version of the reaction can be drawn when the nucleophilic attack of the peroxide originates the O-H bind instead of the lone pair of the oxygen.

In any case, one thing is for sure – epoxidation is a concerted mechanism, meaning that all the bonds are formed and broken simultaneously (recall the old and sweet SN2 mechanism).
There are two arguments we can bring to support the concerted nature of the mechanism.
First, carbocation rearrangements are not observed, and second, is the stereochemistry of the reaction; it is a stereospecific syn-addition. Let’s look at this in a little more detail.
The Stereochemistry of Alkene Epoxidation
The epoxidation is a syn addition, and the stereochemistry of the product is determined based on the structure of the alkene. Cis alkenes give cis epoxides, and trans alkenes give trans epoxides.
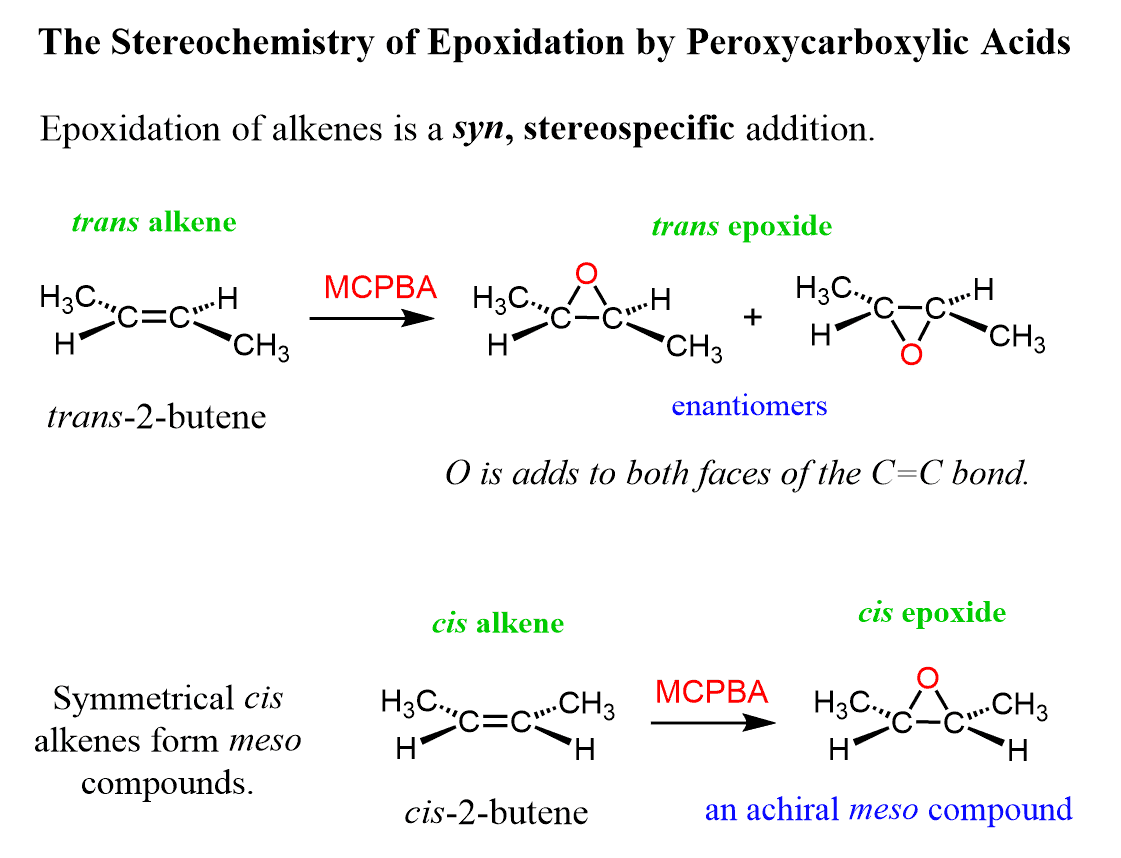
Remember, when the stereochemistry of the product is dictated by the stereochemistry of the substrate, the reaction is said to stereospecific, i.e. it has no choice-cis gives cis, trans gives trans regardless of their stability.
The epoxide can react with the alkene from both faces; therefore, the oxygen can add to either face of the alkene.
As a result, a mixture of enantiomers is obtained when substituted alkenes react with acid peroxides:

You can selectively produce one of the enantiomers in excess by enantioselective epoxidation, for example by Sharpless epoxidation, but we will skip this for now as it is often not needed in an undergraduate course of Organic Chemistry.
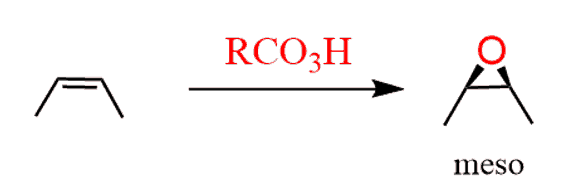
As usual, watch for meso compounds as not every compound with stereogenic centers is going to be chiral. For example, when a symmetrical cis alkene is used, the corresponding meso epoxide is obtained:
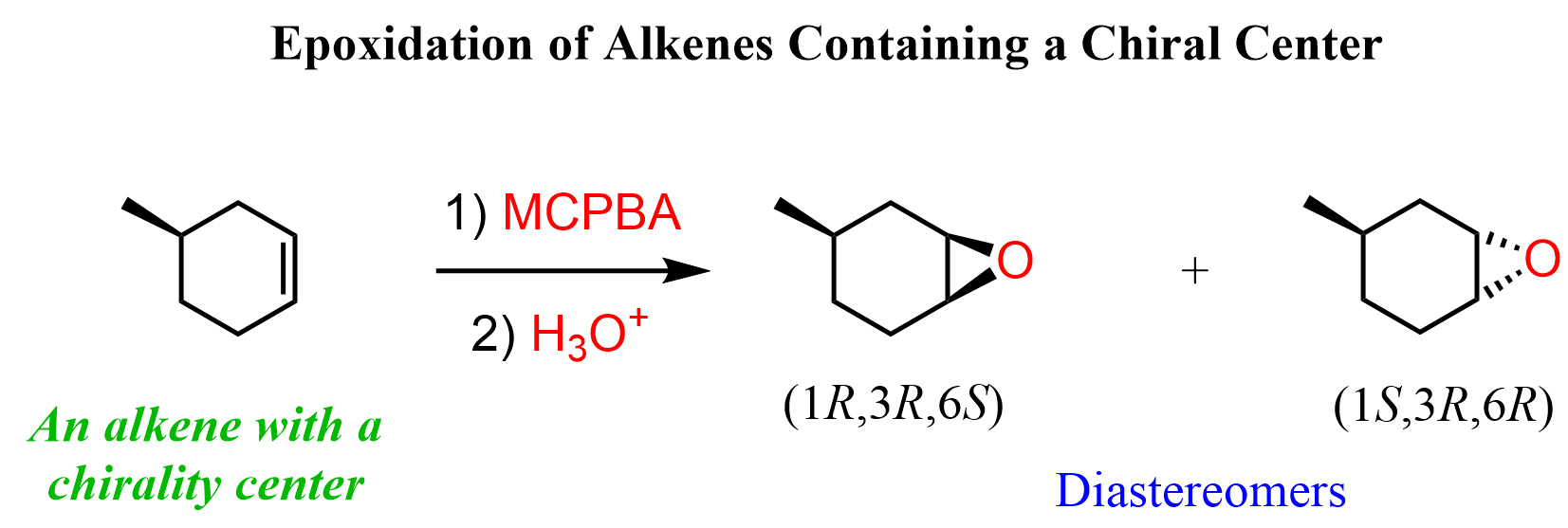
If the alkene contains a chiral center, then the product will have three chirality centers, and therefore, they will be classified as diastereomers:
The Application of Alkene Epoxidation
A question worth addressing here is the use of alkene epoxidation. We need to know that epoxides are very reactive due to a great ring strain, and they react with virtually all the good and poor nucleophiles. What is also important is that the ring-opening reactions of epoxides are regio- and stereoselective which increases the pool of compounds that can be synthesized from them depending on the conditions of the reaction:

For example, by treating an epoxide with water under acidic conditions, we can prepare diols with distinct stereochemical outcomes.
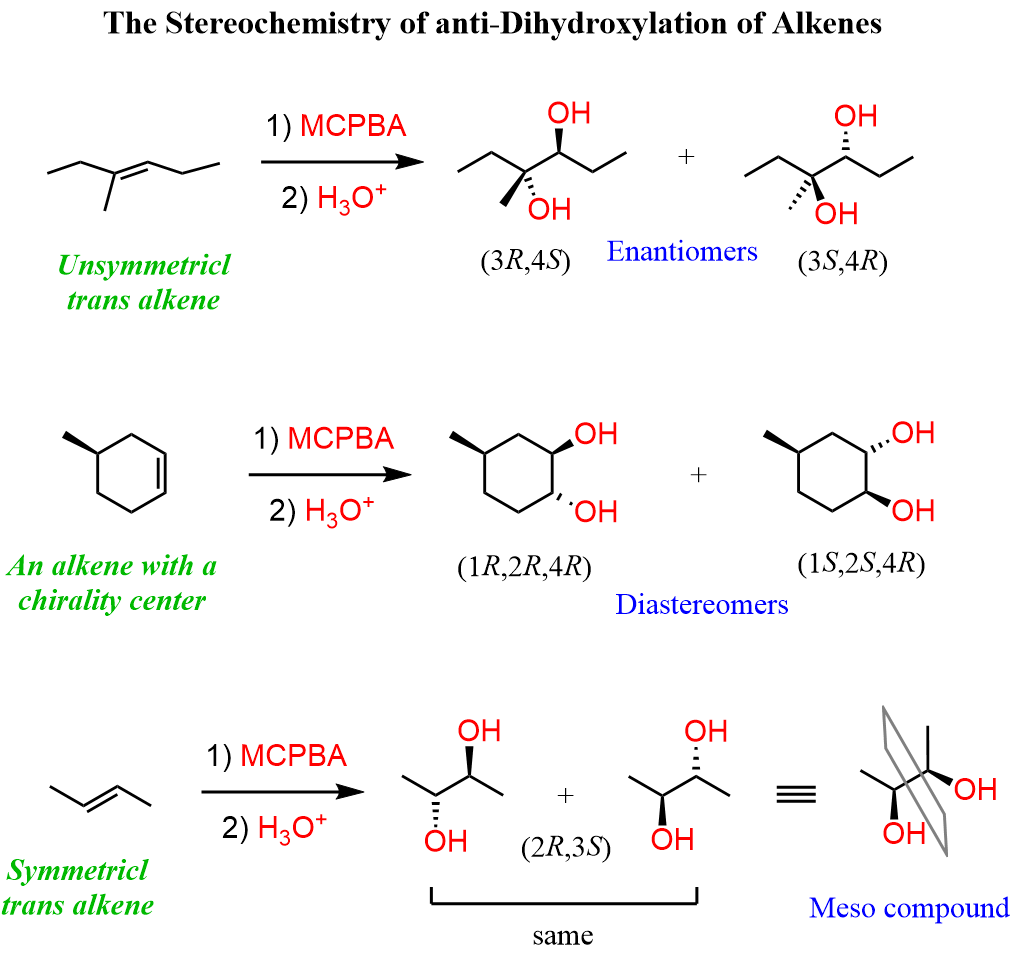
Check the articles “anti-Dihydroxylation of Alkenes” and “Diols from Alkenes” for details about these reactions.
Check Also
- Electrophilic Addition Reactions to Alkenes
- Markovnikov’s Rule
- Markovnikov’s Rule with Practice Problems
- Addition of Water to Alkenes
- Acid-Catalyzed Hydration of Alkenes with Practice Problems
- Rearrangements in Alkene Addition Reactions
- Oxymercuration-Demercuration
- Addition of Alcohols to Alkenes
- Free-Radical Addition of HBr: Anti-Markovnikov Addition
- Hydroboration-Oxidation: The Mechanism
- Hydroboration-Oxidation of Alkenes: Regiochemistry and Stereochemistry with Practice Problems
- Halogenation of Alkenes and Halohydrin Formation
- The Regiochemistry of Alkene Addition Reactions
- The Stereochemistry of Alkene Addition Reactions
- Cis product in an anti Addition Reaction of Alkenes
- Ozonolysis of Alkenes with Practice Problems
- Syn Dihydroxylation of Alkenes with KMnO4 and OsO4
- Anti Dihydroxylation of Alkenes with MCPBA and Other Peroxides with Practice Problems
- Oxidative Cleavage of Alkenes with KMno4 and O3
- Alkene Reactions Practice Problems
- Changing the Position of a Double Bond
- Changing the Position of a Leaving Group
- Alkenes Multi-Step Synthesis Practice Problems
- Alkene Addition Reactions Practice Quiz
