Alkylation of Terminal Alkynes
Many reactions of alkynes are similar to those of alkenes, as they are both π-bond systems that are capable of doing electrophilic addition reactions.
One important difference is the acidity of the alkyne proton. The pKa value for the alkyne protons is ~25 compared to the alkanes (50) and alkenes (44). This is a huge difference, which makes it possible to deprotonate terminal alkynes with strong yet safe-to-handle bases such as the sodium amide or sodium hydride:
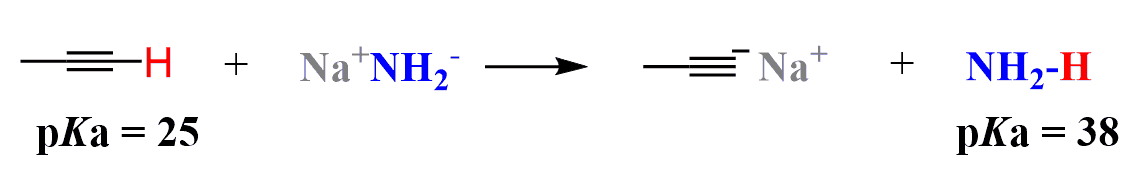
This difference in acidity is a result of the hybridization of the carbon atom bearing the negative charge. The more s-character the orbital has, the more electronegative, thus more stable that carbanion is since it better stabilizes the negative charge.

The resulting acetylide ion is both a strong base and an excellent nucleophile, which is why it is a very important reagent in organic synthesis:

When reacted with sterically unhindered 1o substrates, they give nucleophilic substitution by the SN2 mechanism:
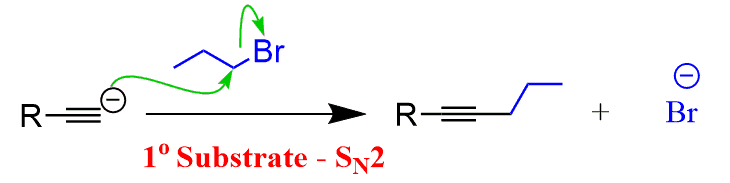
This reaction is one of the first strategies that we learn in Organic synthesis for extending the carbon chain of the substrate.
When reacted with sterically hindered 2o or 3o substrates, they give elimination by the E2 mechanism:

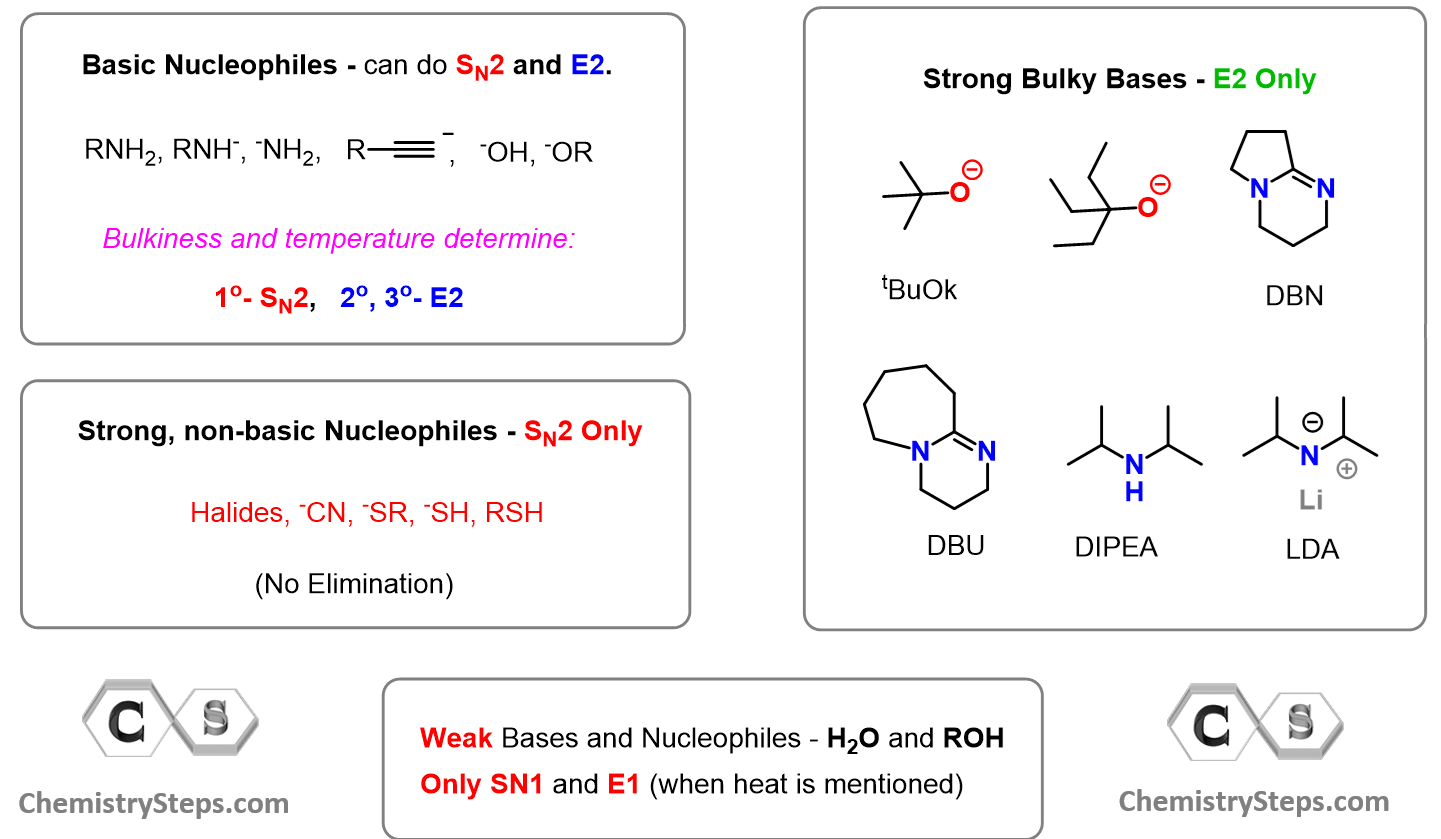
A reminder that good nucleophiles that are also strong bases (basic nucleophiles) perform SN2 substitution with primary alkyl halides, while the E2 mechanism predominates with secondary and tertiary alkyl halides.
Check this article on the competition between SN2 and E2 reactions to refresh these concepts a little bit.
Summary of the Alkylation of Alkynes
Alkynes are more acidic than alkanes and alkenes, which allows us to deprotonate into acetylide ions (alkynide ion for a broader term of any terminal alkyne). Acetylide ions are good nucleophiles and strong bases, making them highly reactive intermediates in organic synthesis.
They readily undergo SN2 substitution with primary alkyl halides to form carbon-carbon bonds, which is a valuable tool for chain extension. However, with secondary and tertiary halides, E2 elimination competes and often predominates.
Acetylide ions also react with epoxides, opening the ring at the less substituted carbon to yield alcohols after protonation.
Always watch out for acid-base side reactions, especially in the presence of protic functional groups like alcohols. In such cases, protecting groups may be necessary.

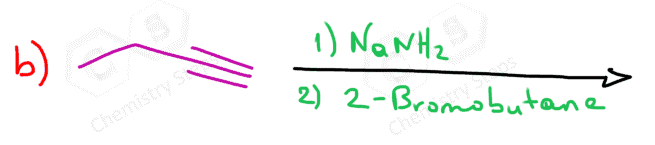
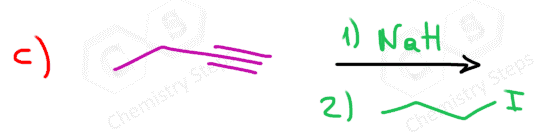
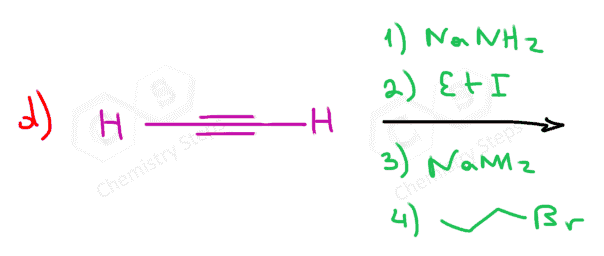
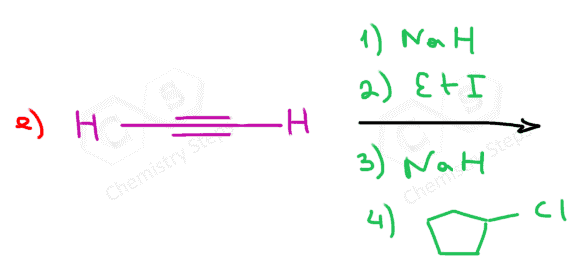

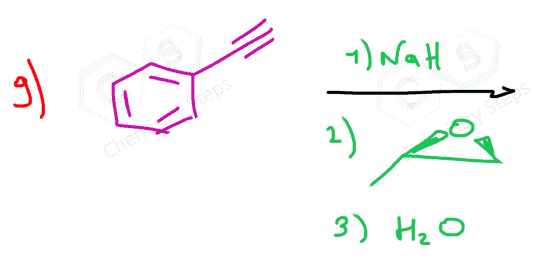
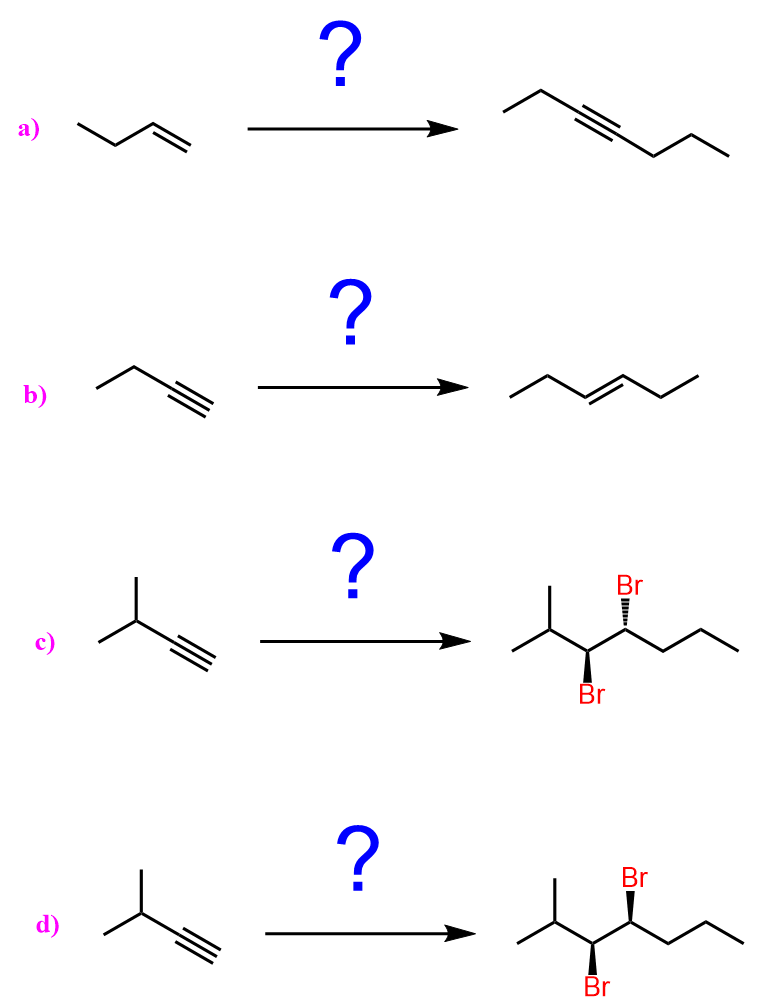
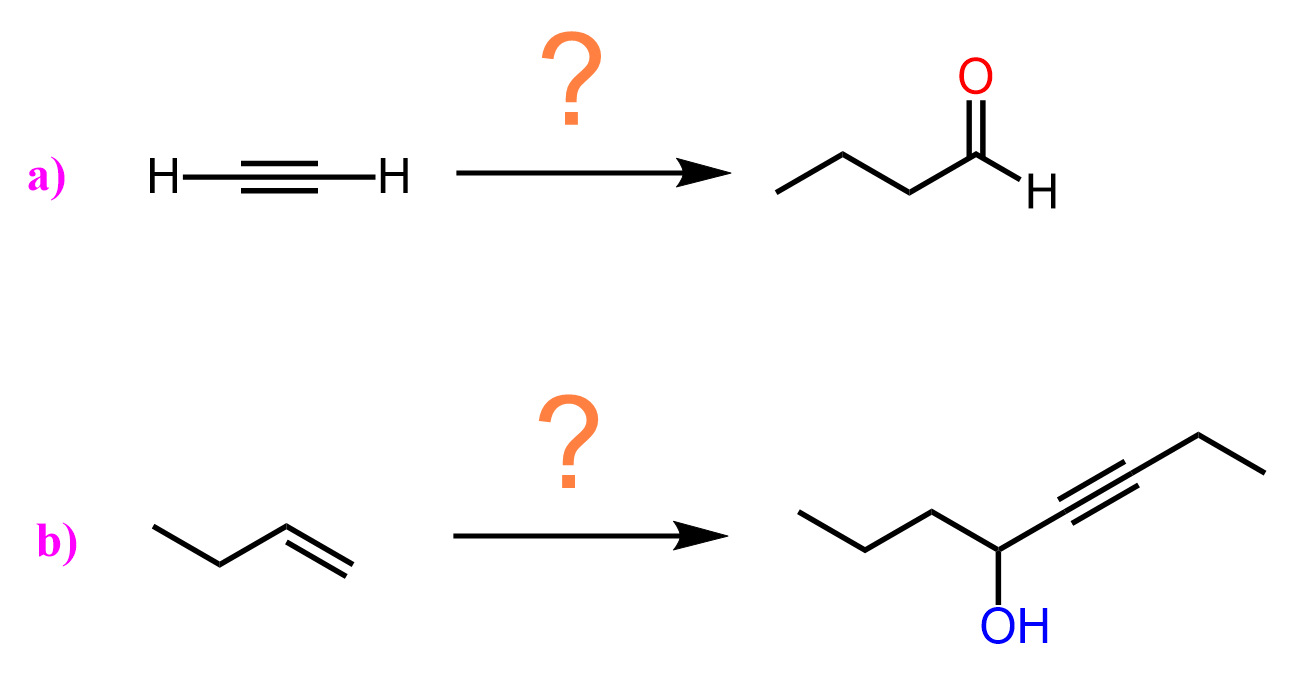
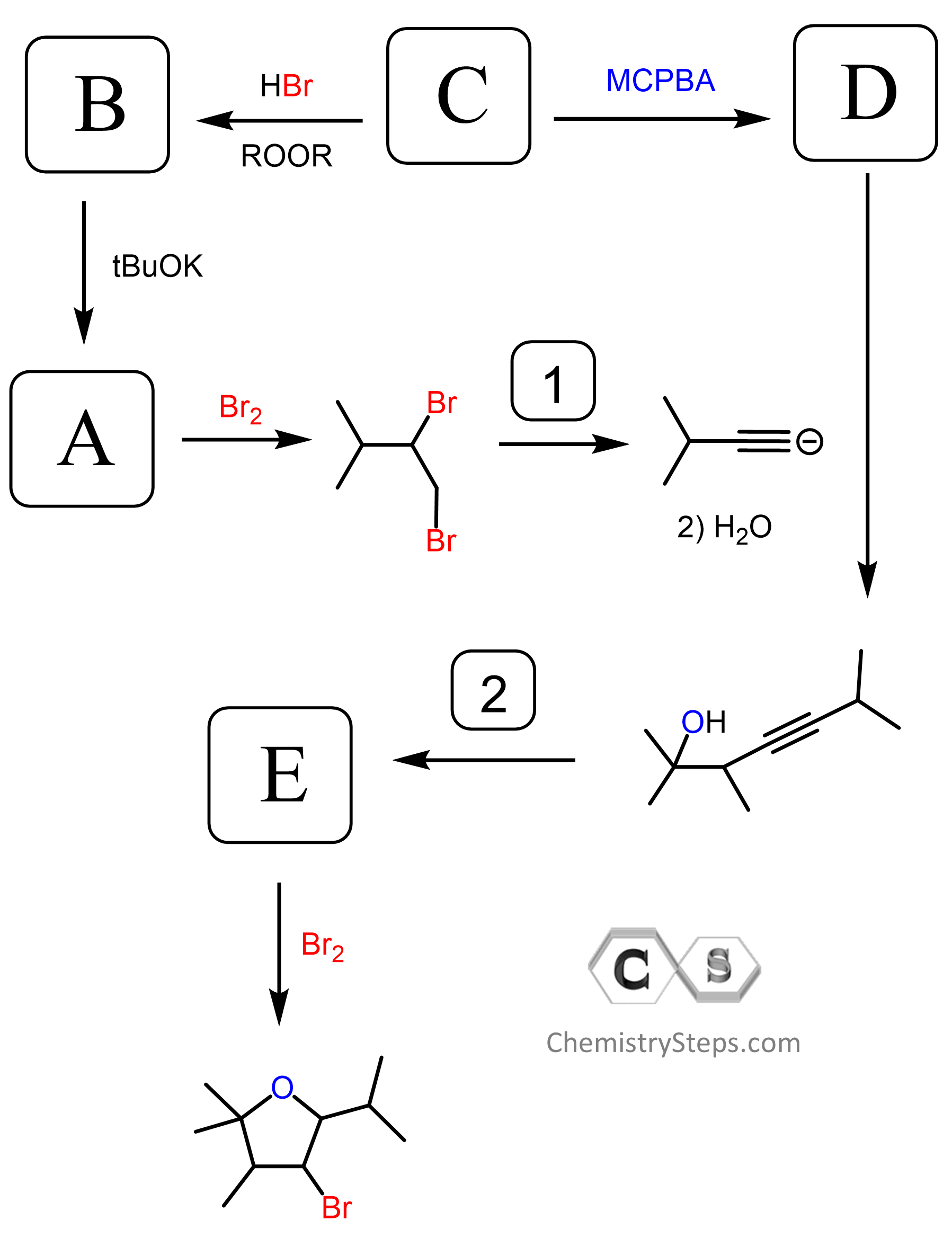
can you show the mechanisms for these? as in the arrows
Yes, they are all shown in the video under the “solution” tab.