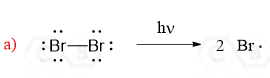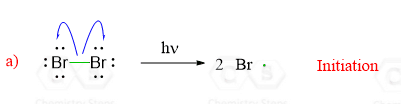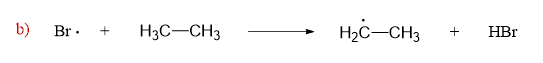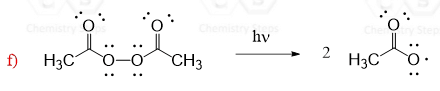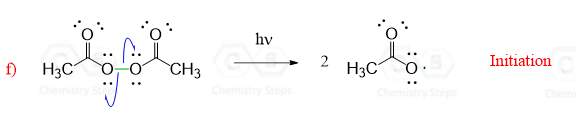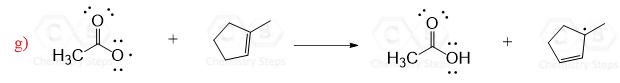Initiation, Propagation, Termination – The Mechanisms
Let’s discuss the chlorination of ethane to illustrate the three important steps of radical halogenation:

Initiation of Radical Halogenation
The reaction starts with homolysis of a σ bond forming two radicals. This is called initiation – the start of the radical chain reactions. We are breaking a bond in the starting materials, so this step requires energy from light or heat:

Notice that unlike most other reactions in organic chemistry such as nucleophilic substitution, elimination or additions, radical reactions are shown by half-headed arrows and one arrow shows movement of one electron.
Half-headed arrows, also known as fishhook arrows, indicate that the σ bond is broken homolitically, i.e. the electrons of the covalent bond are shared between the two atoms.
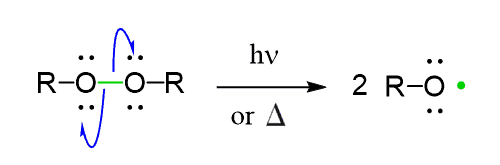
Initiation at moderate temperatures can also be catalyzed by peroxides. One of the features of peroxides is the presence of a weak O-O bond which, upon breaking, forms radicals.
Have you ever wondered why some medicals are sold in dark while others in dark bottles?

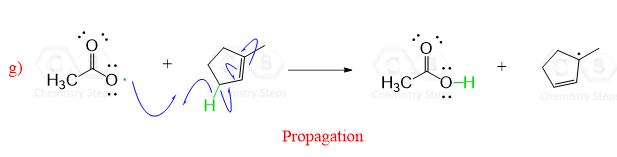
Acyl peroxides are especially useful for this purpose since the resulting radicals are resonance stabilized:
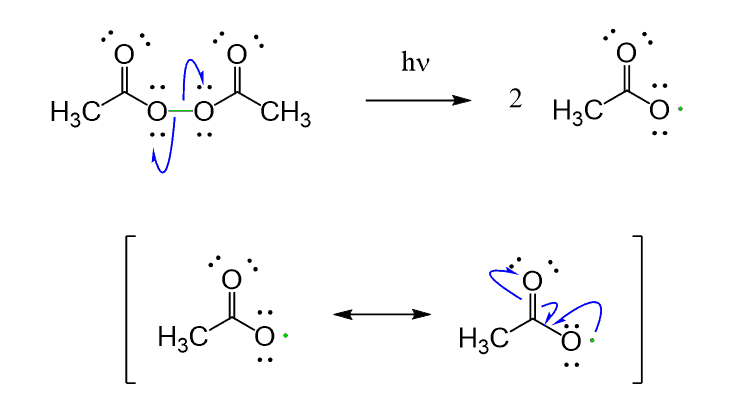
Simple peroxides act in a similar way to produce alkoxyl radicals which are then used in radical reactions:
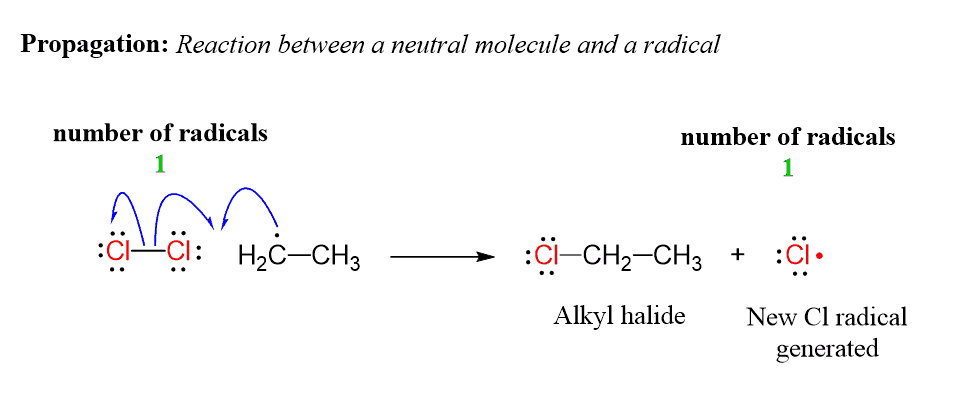
Propagation in Radical Halogenation
The Cl• radicals produced in the initiation step are very unstable. These are the chlorine atoms and you know from general chemistry that halogens exist as diatomic molecules and not atoms.
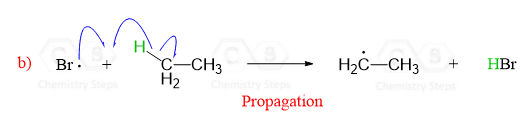
In the next step, these reactive radicals quickly react with ethane by abstracting a hydrogen:

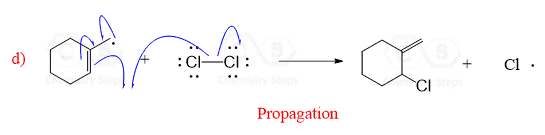
This is a propagation step and what is important in this reaction between a neutral molecule and a radical is that a new radical is created.
The hydrogen abstraction by the halogen radical, is the rate-determining step in radical halogenation.
The alkyl radicals are also very reactive, and they react with the chlorine gas producing a new chlorine radical. Thus, the process continues over and over without a need for supplying energy to perform an initiation again:
This is why radical reactions are classified as chain reactions – only an initial boost is needed to start the reaction and once started, the processes continue repeatedly.
It is also worth mentioning that hydrogen abstraction is different from proton transfer reactions which follow ionic mechanism. Hydrogen abstraction is the movement of H• radical while, proton transfer is the movement of H+ ion:

Mono and Polychlorination in Radical Reactions
The initiation and propagation steps we covered so far represent monochlorination to form chloroethane (ethyl chloride). However, if Cl2 is added in excess or equal quantity, polychlorination is observed.
For example, when equal amounts of methane and chlorine are mixed and irradiated with a light of the appropriate wavelength, the reaction produces a mixture of following products:
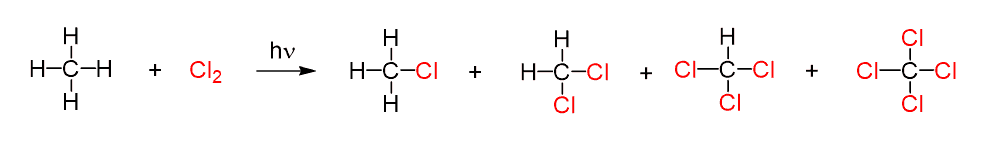
The reason for getting this mixture of mono- and polychlorinated methane is the change of reactant and product concentrations as the reaction proceeds.
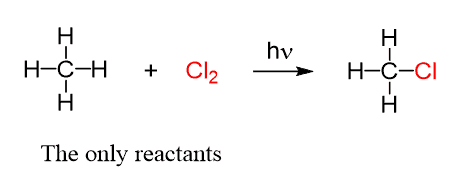
Initially, only chlorine and methane are present, and their reaction produces chloromethane and hydrogen chloride:
However, in the course of the reaction, the concentration of chloromethane in the mixture increases. In addition, chloromethane is more reactive than methane and despite its lower concentration, it starts reacting with chlorine to produce dichloromethane:
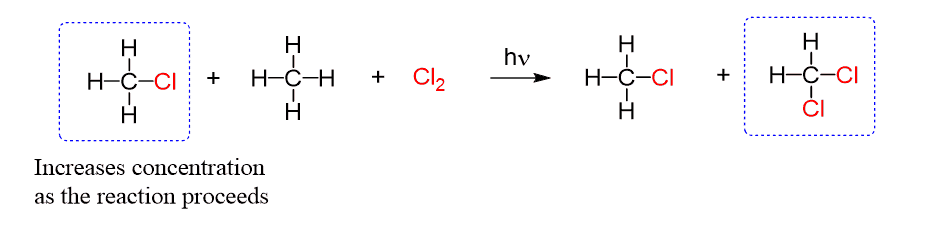
In a similar manner, as the concentration of dichloromethane increases it produced trichloromethane which is then converted into tetrachloromethane.
The chlorination of ethane produces 1,1-dichloroethane and 1,2-dichloroethane, as well as a mixture of more highly chlorinated ethanes:
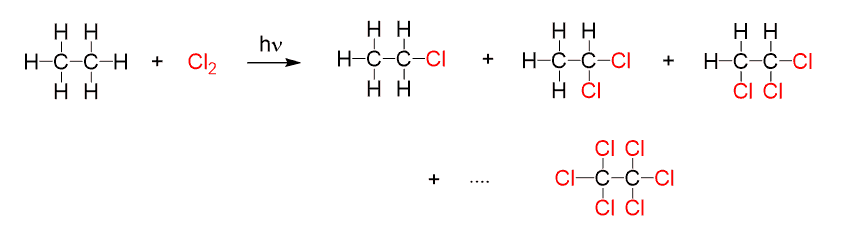
Therefore, in order to achieve monohalogenation, the alkane must always be used in excess. Monohalogenation is important as it allows to perform selective halogenation using bromine and, in general, it is more useful for preparing haloalkanes used in organic synthesis.
There is one good question you might be wondering:
If the Radicals Are so Unstable, Why Don’t They React Together?
The answer to this is they do. In fact, when two radicals meet, they react very fast because of their instability. However, the keyword here is “when” or “if”.
The concentration of radicals is very low compared to the concentration of neutral molecules and they simple do not get a chance to collide. Therefore, the propagation continues repeating the steps thousands of times before eventually radicals meet and react.
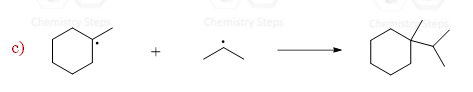
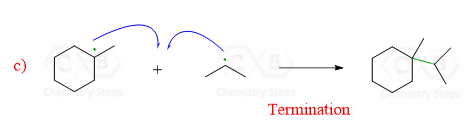
When two radicals react, they start sharing the unpaired electrons and form a new σ bond. This is the termination step which ends the chain of propagation steps:
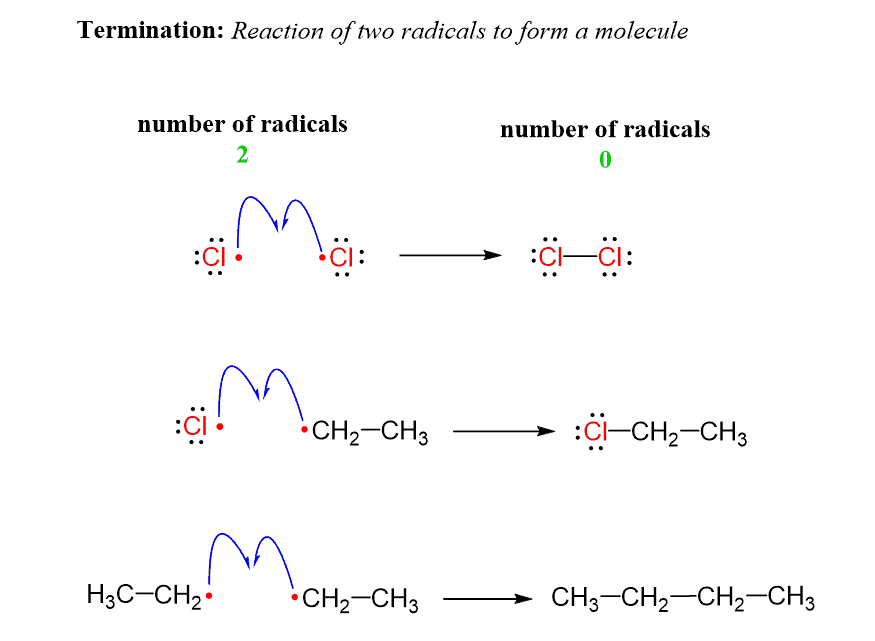
The termination is not restricted to the reaction of specific radicals. Any two radicals reacting to form neutral species represent a termination reaction.
So, termination is the combination of two radicals to form a stable bond and the net result is going from two radicals to no radicals.
This may seem too much if you are going over it first itme but one quick way of identifying these steps in radical halogenation is to keep track of the number of radicals:
- Initiation – goes from 0 radicals to two radicals
- Propagation – goes from 1 radical to two radicals
- Termination – goes from 2 radicals to 0 radicals