In allylic bromination, the Br atom appears on the carbon next to the double bond:

This reaction goes through a radical mechanism, and it is interesting to notice the difference with the aniti-Markovnikov radical bromination:
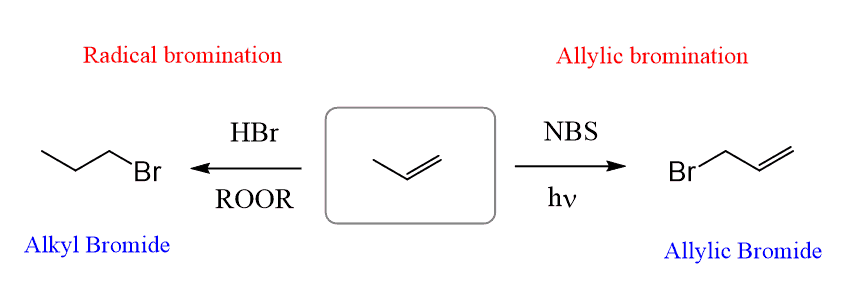
We will discuss why these reactions form different products later. For now, let’s understand how the allylic bromination happens.
First, it is important to mention that allylic radicals are “very stable”. They are even more stable than the tertiary radicals because of resonance stabilization:

This is the driving force of the allylic bromination.
The Mechanism of Allylic Bromination
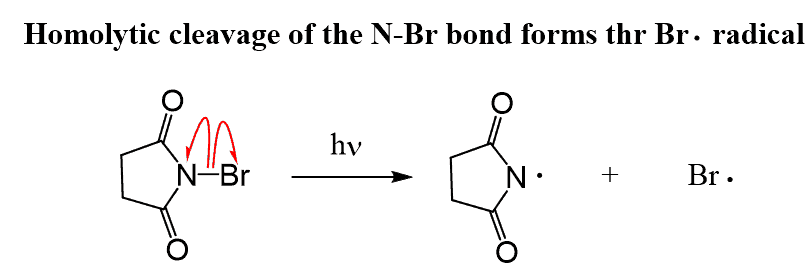
Step 1: The first step of allylic bromination is the homolytic cleavage of the N-Br bond (initiation) of the N-bromosuccinimide (NBS):
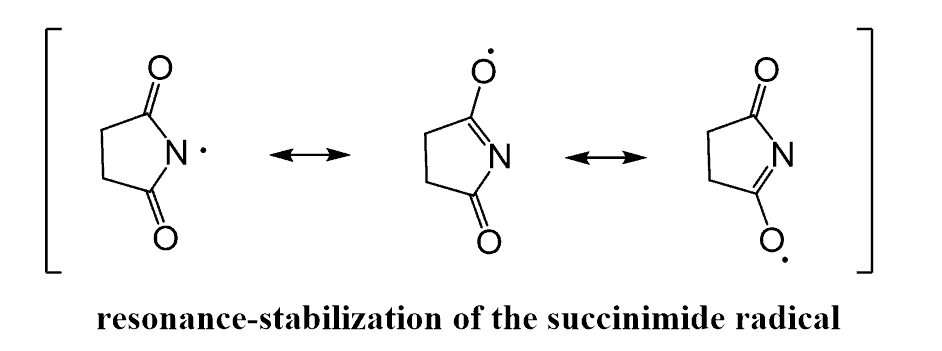
Notice that the imide group can stabilize the radical by two additional resonance structures, which help to initiate the homolysis of the N-Br bond:
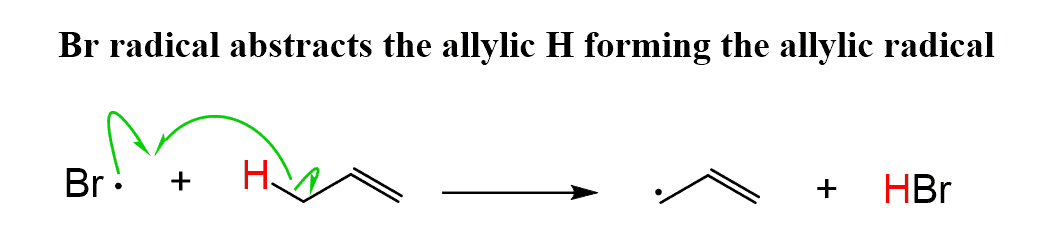
Step 2: After this, the Br radical abstracts an allylic H, forming the corresponding allylic radical:
Step 3: The HBr produced in this step then reacts with NBS, producing Br2 in low concentration.

Step 4: In the next step, the Br2 is then quickly captured by the allylic radical, thus keeping the concentration of HBr and Br2 at a minimum, suppressing the competing electrophilic addition to the double bond.
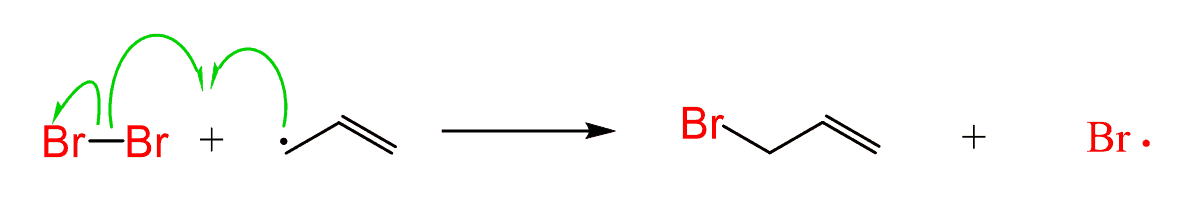
The process repeats until the termination and consumption of the reactant(s).
Looking at the last step, there is one question we didn’t address here: where is the Br2 coming from?
The source of Br2 is the NBS, which, besides producing the Br radical, generates a low concentration of Br2.
Allylic Bromination vs Addition to the Double Bond
Now, let’s go back and figure out this question: why does the Br radical generated by NBS not add to the double bond like in the anti-Markovnikov bromination?

The answer is it does! However, because the concentration of HBr is low (remember, HBr is needed to supply the hydrogen and convert the radical into alkyl bromide), the addition reaction reverses and proceeds by allylic bromination:

The regiochemistry of Allylic Bromination
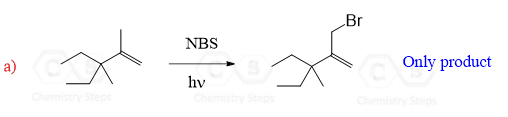
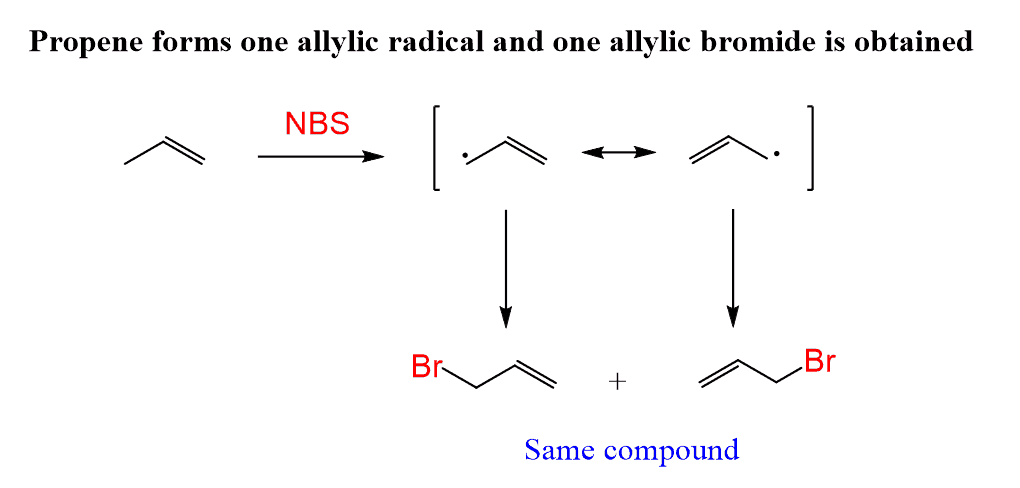
The example we discussed above was based on the simplest alkene with an allylic position (propene). As a result, only one product could be obtained since the two resonance structures are superimposable mirror images:
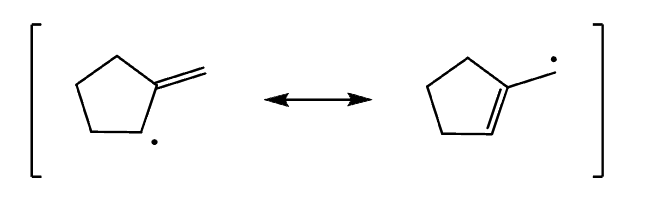
However, if the radical stabilization does not result in identical allylic radicals, a mixture of allylic bromides is obtained:

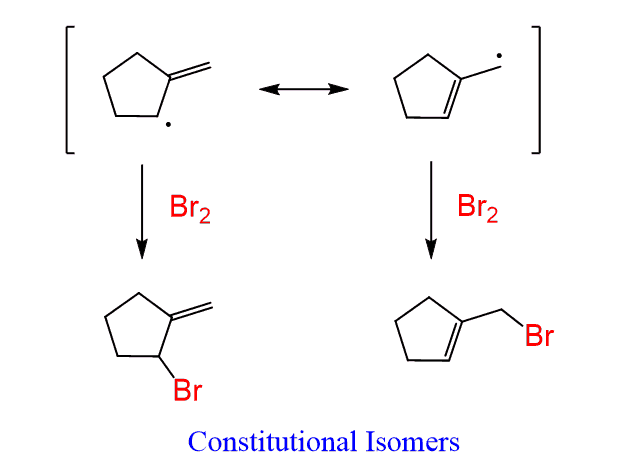
Notice that both allylic resonance structures contribute to the formation of the two constitutional isomers. So, whenever you are asked to determine the products of allylic bromination, draw both resonance forms and place the Br atoms accordingly.
For example, predict the products of allylic bromination of the following alkene:
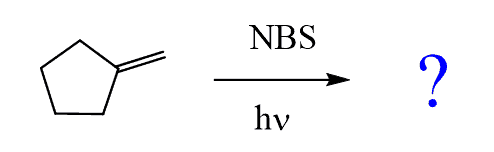
Step 1: Draw the allylic radical:

Step 2: Draw the resonance structures of the radical:
Step 3: Add the Br to the allylic radical of each resonance structure:
Stereochemistry of Allylic Bromination
There is no stereochemical control on the allylic bromination. Just like any radical (or carbocation) reaction, whenever possible, both R and S configurations of the radical carbon are formed.
For example, 1-butene, which we discussed earlier, forms three products in total:

One of the radicals forms a mixture of enantiomers, while the other one can only form one product. This product is a constitutional isomer of the two enantiomers.